Question: Let r 1 (r 2 ) point to a line element ds 1 (ds 2 ) of a closed loop C 1 (C 2 )
Let r1 (r2) point to a line element ds1 (ds2) of a closed loop C1 (C2) which carries a current I1 (I2). Experiment shows that the force exerted on I1 by I2 is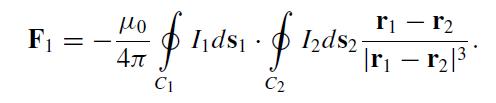
(a) Show that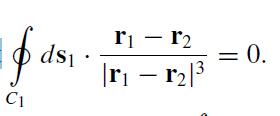
(b) Use (a) to show that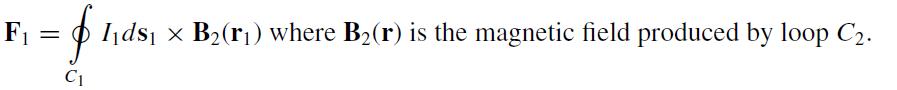
F = - Ijds $12ds2 Mo 4 C1 C2 11 - 12 |r r| -
Step by Step Solution
3.50 Rating (150 Votes )
There are 3 Steps involved in it
a b We use the identity Substituting this equation into the given expr... View full answer

Get step-by-step solutions from verified subject matter experts


