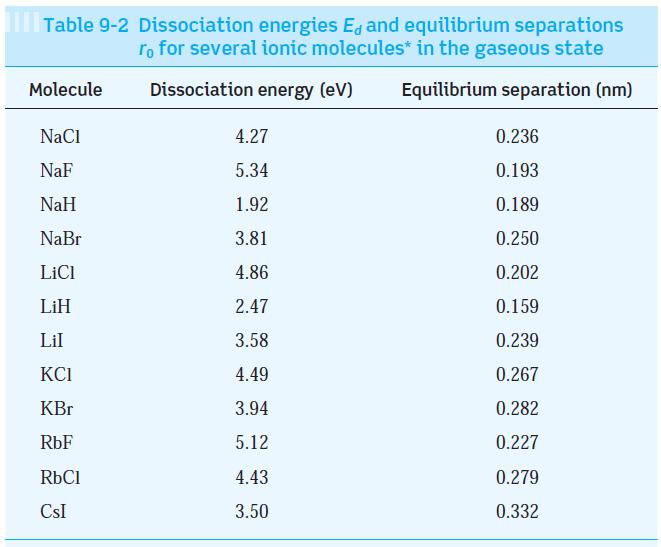
Note in Table 9-2 that the equilibrium separation of the KBr and RbCl molecules is very nearly
Question:
Note in Table 9-2 that the equilibrium separation of the KBr and RbCl molecules is very nearly equal. Compute the exclusion-principle repulsion for these molecules.
Transcribed Image Text:
Table 9-2 Dissociation energies E, and equilibrium separations ro for several ionic molecules* in the gaseous state Dissociation energy (eV) Equilibrium separation (nm) Molecule NaCl NaF NaH NaBr LiCl LiH Lil KCI KBr RbF RbCl CSI 4.27 5.34 1.92 3.81 4.86 2.47 3.58 4.49 3.94 5.12 4.43 3.50 0.236 0.193 0.189 0.250 0.202 0.159 0.239 0.267 0.282 0.227 0.279 0.332
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
For KBr Uc 1440eVnm 0282nm E 394...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
The equilibrium separation of CsF is 0.2345 nm. If its bonding is 70% ionic, what is its electric dipole moment.
-
The equilibrium separation of H atoms in the H2 molecule is 0.74nm (Fig 29-8). Calculate the energies and wave-lengths of photons for the rotational transitions (a) L = 1 to L = 0, (b) L = 2 to L =...
-
The equilibrium separation of the HF molecule is 0.0917 nm and its measured electric dipole moment is 6.40 10-30 C m. What percentage of the bonding is ionic?
-
Read the Comp & Ben Case Study and answer the following question: To the degree job growth (and increased car sales that come from m costs) is based on two tier-wage structures, how sustainable...
-
Kent Tessman, manager of a Dairy Products Division, was pleased with his divisions performance over the past three years. Each year, divisional profits had increased, and he had earned a sizable...
-
What role do you think the individual needs of people play in building a successful company? LO3
-
Redshifts of quasi-stellar objects. Refer to the Journal of Astrophysics & Astronomy (Mar./Jun. 2003) study of redshifts in quasi-stellar objects presented in Exercise 11.27 LO9 (p. 627). Recall that...
-
Selected accounts from the adjusted trial balance for Louise's Gourmet Shop as of March 31, 2011, the end of the current fiscal year. The merchandise inventory for Louise's Gourmet Shop was $38,200...
-
Match 1)Master budget 2)Sales Budget 3)Production Budget 4)Direct Material Purchases Budget 5) Direct Labor Budget 6) Manufacturing Overhead Budget 7) Budgeted Income Statement 8) Capital...
-
Robert J. and Sally L. Jones are married and file a joint return. Robert is self-employed as a dentist, and Sally is a college professor. Robert and Sally have three children. The oldest is Vince who...
-
An early method testing Maxwells theoretical prediction for the distribution of molecular speeds is shown in Figure 8-34. In 1925 Otto Stern used a beam of Bi 2 molecules emitted from an oven at 850...
-
If the Sun were to become cooler (without changing its radius), the energy density at the surface would decrease according to Equation 8-56. Suppose the Suns temperature were to decrease by 5...
-
What does the term break-even point mean? Name the two ways it can be measured.
-
A new partner C is invited to join in the AB partnership. Currently, A's and B's capital are $540,000 and $100,000, respectively. According to their profit and loss sharing contract, partner A and B...
-
The two tanks shown are connect through a mercury manometer. What is the relation between ???? and ? water Az water Ah
-
1. After reading about the types of rights that prisoners have while incarcerated, which of these rights, if any, should be reduced or diminished? Why? 2. In the same way, what rights do you believe...
-
According to the Socratic view of morality summarized by Frankena, is a person brought up by immoral parents in a corrupt society capable of making correct moral judgements? Why or why not? Do you...
-
Loma Company manufactures basketball backboards. The following information pertains to the company's normal operations per month: Output units15,000 boards Machine-hours4,000 hours Direct...
-
What are the registers? What are their functions?
-
Will the prediction interval always be wider than the estimation interval for the same value of the independent variable? Briefly explain.
-
A particle starts from x 0 = 10 m at t 0 = 0 s and moves with the velocity graph shown in FIGURE EX2.6. a. Does this particle have a turning point? If so, at what time? b. What is the objects...
-
A particle starts from x 0 = 10 m at t 0 = 0 s and moves with the velocity graph shown in FIGURE EX2.6. a. Does this particle have a turning point? If so, at what time? b. What is the objects...
-
FIGURE EX2.7 is a somewhat idealized graph of the velocity of blood in the ascending aorta during one beat of the heart. Approximately how far, in cm, does the blood move during one beat? Figure Ex...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


