The PI3K/AKT signaling pathway is aberrant in a wide variety of cancers. In soft-tissue sarcoma (STS) cells,
Question:
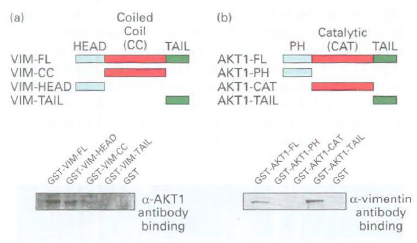
a. To map the vimentin and AKT interaction domains, the vimentin and AKTl full-length and fragment constructs indicated below were expressed as GST-fusion proteins. Each of the GST-fusion constructs bound to glutathione beads was used to pull down associated proteins from crude STS cell lysates. What did the Western blot analysis of the pellet using an AKTl antibody reveal about the AKT-binding domain in vimentin? What did analysis of the pellet using a vimentin antibody reveal about the vimentin-binding domain in AKTl?
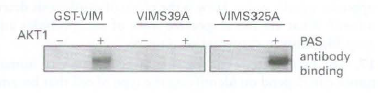
b. AKTl is a kinase and therefore likely to phosphorylate vimentin. Sequence analysis revealed that serines (S) at positions 39 and 325 in vi men tin were likely sites for AKT 1 phosphorylation. To test whether one or both of these sites is phosphorylated by AKTl, each site was mutated to alanine (A), which cannot be phosphorylated. Each alaninemutated
vimenrin was mixed with AKTl and tested for phosphorylation by Western blot analysis using an antibody that reacts with AKTl-phosphorylated serines (PAS antibody). Which site(s) does AKTl phosphorylate?
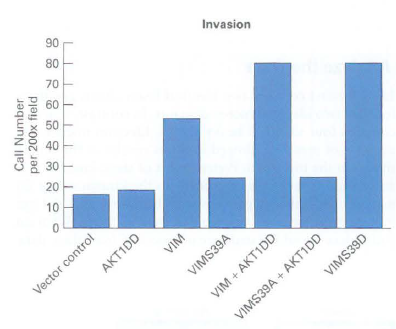
c. The propensity of cancer cells to metastasize can be measured with an invasion assay in which cells migrate through a filter coated with extracellular matrix (ECM) proteins. The more cells migrating through the ECM, the higher the propensity for metastasis. The invasion assay was used to monitor effects of expression of a permanently active AKTl (AKTl DD) mutation and overexpression of wild-type vimentin, a vimemin mutation that cannot be phosphorylated by AKTl (VIMS39A), and a phosphoniimetic vimentin mutation (VIMS39D) in which mutation of S39 to an aspartate residue (D) mimics phosphorylation of the serine. What effect does vimentin overexpression and phosphorylation have on cell migration?
Step by Step Answer:

Molecular Cell Biology
ISBN: 978-1429234139
7th edition
Authors: Harvey Lodish, Arnold Berk, Chris A. Kaiser, Monty Krieger, Anthony Bretscher, Hidde Ploegh, Angelika Amon, Matthew P. Scott





