In thermodynamics, you learned that the saturation pressure for a one component vaporliquid equilibrium can be computed
Question:
In thermodynamics, you learned that the saturation pressure for a one component vapor–liquid equilibrium can be computed using an integral construction from the phase diagram, where the area “under” or “over” the curve needs to be equal. Using a departure function method, the saturation pressure at 395 K for the van derWaals fluid in Problem 5 was 1.404 886 MPa. Write a MATLAB program that uses Gauss quadrature to see if this result also agrees with the integral construction.
Problem 5
If I want to compute the integral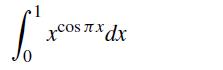
using two point Gauss quadrature, at what values of x do you evaluate the function xcos πx?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Numerical Methods With Chemical Engineering Applications
ISBN: 9781107135116
1st Edition
Authors: Kevin D. Dorfman, Prodromos Daoutidis
Question Posted:





