Compound A (C 8 H 15 Cl) exists as a racemic form. Compound A does not react
Question:
Compound A (C8H15Cl) exists as a racemic form. Compound A does not react with either Br2 or dilute aqueous KMnO4. When A is treated with magnesium in dry ether followed by aqueous acid and the mixture is separated by gas chromatography, two fractions, B and C, are obtained. The components of both fractions have the formula C8H16. Fraction B consists of a racemic form and can be resolved. Fraction C cannot be resolved. Treating A with sodium ethoxide in ethanol converts A into D (C8H14). Hydrogenation of D using a platinum catalyst yields C. Ozonolysis of D followed by treatment with dimethyl sulfide yields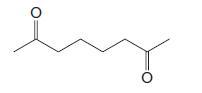
Propose structures for A, B, C, and D, including, where appropriate, their stereochemistry
Step by Step Answer:

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder





