Predict the products from each of the following reactions. (a) (b) (c) (d) (e) (f) (g) (1)
Question:
Predict the products from each of the following reactions.
(a)
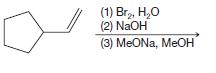
(b)
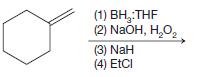
(c)
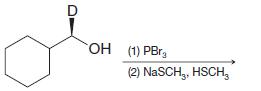
(d)
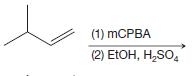
(e)
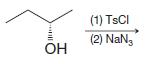
(f)
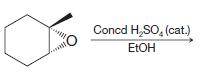
(g)
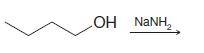
Transcribed Image Text:
(1) Br₂, H₂O (2) NaOH (3) MeONa, MeOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
A sample of copper sulphate pentahydrate contains 3.782 g of Cu. How many grams of oxygen are in the sample? 1) 0.952 g 1 2) 3.809 g 3) 4.761 g 4) 8.576 g
-
Predict the products from each of the following reactions. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) Hl (excess) Hi H,so,,H20 O MeONa O MeOH, cat. H2SO (1) EtSNa (2) H2o HCl (1 equiv.) MeONa (1)EtON...
-
1. [10] Let A = 2 4 a b c d e f g h i 3 5, B = 2 4 d + 5g e+ 5h f+ 5i a 2g b 2h c 2i g h i 3 5, C = 2 4 2g 2h 2i 3d 3e 3f 1a 1b 1c 3 5. Suppose that det A = 5. Find det B = , det C = , and det (AC) =
-
Two moles of an ideal monatomic gas go through the cycle abc. For the complete cycle, 800 J of heat flows out of the gas. Process ab is at constant pressure, and process bc is at constant volume....
-
How are communicable diseases devastating human and economic development in some poor nations?
-
Refer to the facts in problem 2. Compute the state income tax savings if Oldham could relocate its personnel so that payroll expense in State M increased to $1,900 (thou-sand) and payroll expense in...
-
Explain what directional shopping is and where it fits in the buying calendar. LO.1
-
Wyrick Inc. had the following transactions pertaining to investments in common stock . Jan. 1 Purchased 2,500 shares of Murphy Corporation common stock (5%) for $140,000 cash plus $2,100 brokers...
-
The following information is available for Lock-Tite Company, which produces special-order security products and uses a job order costing system. April 30 May 31 $ $43,000 9,700 62,000 59,000 18,900...
-
Jim Harrod knew that service, above all, was important to his customers. Jim and Becky Harrod had opened their first store in Omaha, Nebraska in 1997, Harrod's carried a full line of sporting goods...
-
Draw a free-energy diagram (reaction progress versus free energy) for each of the following reactions. Be sure to label the axes, transition state(s), the energy of activation for each step, and H,...
-
Verizon Communications, Inc. is one of the worlds largest providers of communication services. The following information, taken from the companys annual reports, is available for the years 2018,...
-
Analyze the characteristics of the different types of shopping centers.
-
As a new principal, I assigned a teacher to a different grade for the coming year. I did not expect to cause the anxiety it did. The teacher first came to me in tears and begged for her assignment to...
-
Peruse the following websites to learn about the different ways of categorizing leadership. 1. https://www.businessnewsdaily.com/9789-leadership-types.html 2....
-
Making Consumer Choices The Espresso Machine (25 points) In real life, you must often make choices about whether to buy something pre-made or make it yourself. There are many things to consider:...
-
1) Read over the article/case and summarize what it is referring to in your own words. 2) What type of leadership traits can you describe in the case study? Use materials both from the handout and...
-
After reading or watching, https://smallbusiness.chron.com/internal-analysis-important-80513.html https://www.indeed.com/career-advice/career-development/internal-analysis...
-
How does HRM affect all supervisors?
-
The cost curve for the city water supply is C(Q) = 16 + 1/4 Q2, where Q is the amount of water supplied and C(Q) is the cost of providing Q acre-feet of water. (An acre-foot is the amount of water...
-
While working in the chemical stockroom, you discover an unlabeled bottle containing a liquid compound. You carefully smell the liquid and discover that it has a fishy odor. What functional group do...
-
Turpentine, obtained from pine trees, is composed primarily of ?-pinene and ?-pinene, Explain whether you expect turpentine to mix with water. If a point dissolves in turpentine, what does this...
-
The structure of a typical fat is shown here. Estimate the energy content of fat compared to the other compounds discussed in the Focus On box on p. 146 and explain yourreasoning. CH OC(CH) 16CH3 O...
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


