a. List the following esters in order of decreasing reactivity toward hydrolysis: b. How would the rate
Question:
a. List the following esters in order of decreasing reactivity toward hydrolysis:
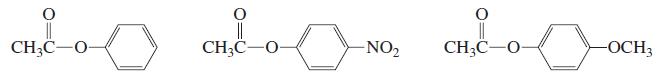
b. How would the rate of hydrolysis of the para-methylphenyl ester compare with the rate of hydrolysis of these three esters?
Transcribed Image Text:
CH3C-O- || CH;C-O- CH3C-O- -NO2 -OCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
A the rate of hydrolysis is highest for p nitrophenyl Aceto ester and the...View the full answer

Answered By

Raju Mandal
From my childhood , i was good in studying. I have completed my 10th exam from sagarpara high school and securing 1st in my batch.Then i have complete 12th standard from krishnath college school.i have completed my BSc in chemistry from krishnath college and my rank was 2nd in my college and 5th in kalyani university.Now i aam studying Master in school of chemistry , university of hyderabad
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
List the following amides in order of decreasing reactivity toward acid-catalyzed hydrolysis: NO, CH CNH- CH CNH CH CNH NO2 CH,CNH
-
List the following dienes in order of decreasing reactivity in a DielsAlder reaction:
-
List the following alcohols in order of decreasing rate of dehydration in the presence of acid: CH3 CH2OH CH3
-
Whitmore Company issued $500,000 of 5-year, 8% bonds at 97 on January 1, 2020. The bonds pay interest annually. Instructions a. 1. Prepare the journal entry to record the issuance of the bonds. 2....
-
Infrared spectra are customarily recorded on a transmittance scale so that weak and strong bands can be displayed on the same scale. The region near 2 000 cm -1 in the infrared spectra of compounds A...
-
Interpreting common-size income statements. The Coca-Cola Company (Coke) and PepsiCo dominate the non-alcoholic beverage segment in the United States. Most beverage manufacturing involves adding...
-
Game performance of water polo players. Refer to the Biology of Sport (Vol. 31, 2014) study of the physiological performance of top-level water polo players, Exercise 11.24 (p. 626). Recall that the...
-
An article in Nuclear Engineering International (February 1988, p. 33) describes several characteristics of fuel rods used in a reactor owned by an electric utility in Norway. Measurements on the...
-
36. Please answer the following questions: a) Consider which of the three buses is the most attractive option on financial grounds. State clearly any assumptions that you need to make. b) Repeat the...
-
Roberts Co. will borrow $250,000 on June 30th, 2021. The CFO is trying to decide whether to accept principal and interest payments over the term of the loan or to pay interest only with a balloon...
-
a. Two amides are obtained from the reaction of acetyl chloride with a mixture of ethylamine and propylamine. Identify the amides. b. Only one amide is obtained from the reaction of acetyl chloride...
-
D. N. Kursanov, a Russian chemist, proved that the bond that is broken in the hydroxideion- promoted hydrolysis of an ester is the acyl C-O bond, rather than the alkyl C-O bond, by studying the...
-
Arturo, a calendar year taxpayer, paid $16,000 in medical expenses and sustained a $20,000 casualty loss in 2013. He expects $12,000 of the medical expenses and $14,000 of the casualty loss to be...
-
Gulf Shore Lawn and Garden Maintenance provides two general outdoor services: lawn maintenance and garden maintenance. The company charges customers $18.0 per hour for each type of service, but lawn...
-
Two level sections of an east highway (G=0) are to be connected. Currently, the two sections of highway are seperated by a 4000-ft (horizontal distance), 2% grade. The westernmost section of highway...
-
A solution contains 2 x 10-3 moles Ca2+/L and 3 x 10-4 moles Mg2+/L. Given the formation constants for CaEDTA2- and MgEDTA2- of 1010.6 and 108.7, respecively, calculate: 1) Concentration of MgEDTA2-...
-
The direct material (DM) price variance is $2,650 favorable and the DM usage variance is $3,000 unfavorable. The budgeted amount of DM for each unit of product is 2 lbs. to be purchased at the...
-
On January 1, 2023, AMI Corporation purchased the non-cash net assets of Oriole Ltd. for $8,399,900. Following is the statement of financial position of Oriole Ltd. from the company's year- end the...
-
A region R is shown. Decide whether to use polar coordinates or rectangular coordinates and write R f(x, y) dA as an iterated integral, where f is an arbitrary continuous function on R. yA 1 R -1 1
-
One hundred pounds of water at atmospheric pressure are heated from 60F to 200F. What is the enthalpy change? The internal energy change? Why is the difference between the internal energy change and...
-
The following compound can become protonated on any of the three nitrogen atoms. One of these nitrogens is much more basic than the others, however. (a) Draw the important resonance forms of the...
-
Circle any lone pairs (pairs of nonbonding electrons) in the structures you drew for Problem 1-3. In problem (a) N2 (b) HCN (c) HONO (d) CO2 (e) CH3CHNH (f) HCO2H (g) C2H3CI (h) HNNH
-
In the following sets of resonance forms, label the major and minor contributors and state which structures would be of equal energy. Add any missing resonance forms. (a) (b) (c) (d) (e) CH,_...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


