D. N. Kursanov, a Russian chemist, proved that the bond that is broken in the hydroxideion- promoted
Question:
D. N. Kursanov, a Russian chemist, proved that the bond that is broken in the hydroxideion- promoted hydrolysis of an ester is the acyl C-O bond, rather than the alkyl C-O bond, by studying the reaction of the following ester with HO-/H2O:
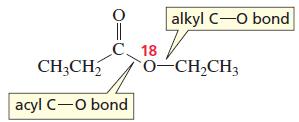
a. Which of the products contained the 18O label?
b. What product would have contained the 18O label if the alkyl C-O bond had broken?
Transcribed Image Text:
alkyl C-o bond CH,CH, C 18 o-CH,CH3 acyl C-O bond
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 36% (11 reviews)
a As the acyl CO bond is broken in the hydroxide ionpr...View the full answer

Answered By

Shakil Ahmed
I hold a Master's degree in Chemistry. I have a special interest in organic and inorganic chemistry. I have worked as a teaching assistant for undergraduate students for a semester. I focus on strengthening the basic concept first as it will help throughout.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The intermediate shown here is formed during the hydroxide-ion-promoted hydrolysis of the ester group. Propose a mechanism for the reaction. - 213 H20 N 0 CH CH 0 N o CH3
-
Early chemists could envision several possible mechanisms for hydroxide-ion-promoted ester hydrolysis: 1. a nucleophilic acyl substitution reaction 2. An S N 2 reaction 3. An S N 1 reaction Devise an...
-
The polymer shown here is synthesized by hydroxide ion-promoted hydrolysis of an alternating copolymer of para-nitrophenyl methacrylate and acrylate. a. Propose a mechanism for the formation of the...
-
A childs grandparents purchase a $10,000 bond fund that matures in 18 years to be used for her college education. The bond fund pays 4% interest compounded semiannually. How much will the bond fund...
-
The metal ion indicator xylenol orange (Table 11-3) is yellow at pH 6 ( max = 439 nm). The spectral changes that occur as VO 2+ is added to the indicator at pH 6 are shown here. The mole ratio VO 2+...
-
Spreadsheet analysis of transactions and preparation of income statement and balance sheet. Refer to the information tor Moulton Corporation as of December 31, Year 12 in Problem 29 of Chapter 2....
-
Sound waves from a basketball. Refer to the American Journal of Physics (June 2010) study of sound waves in a spherical cavity, Exercise 11.31 (p. 629). You fit a straight-line model relating sound...
-
For the year ended December 31, 2011, Fidelity Engineering reported pretax accounting income of $977,000. Selected information for 2011 from Fidelity's records follows: Interest income on municipal...
-
Problem 9-25 Portfolio Return (LG9-7) At the beginning of the month, you owned $6,000 of General Dynamics, $5,000 of Starbucks, and $9,000 of Nike. The monthly returns for General Dynamics,...
-
4. Sleekfon and Sturdyfon are two major cell phone manufacturers that have recently merged. Their current market sizes are as shown in Table 5-9. All demand is in millions of units. Sleekfon has...
-
a. List the following esters in order of decreasing reactivity toward hydrolysis: b. How would the rate of hydrolysis of the para-methylphenyl ester compare with the rate of hydrolysis of these three...
-
An oil obtained from coconuts is unusual in that all three fatty acid components are identical. The molecular formula of the oil is C 45 H 86 O 6 . What is the molecular formula of the carboxylate...
-
Differentiate between direct and indirect costs of bankruptcy. Which of the two is generally more significant?
-
TST102 Fundamentals of Test Evaluation Lesson 17 - Assignment Assignment 1: Developmental Test Planning You are designing a developmental test to verify that the SRAW safe-arm device (SAD) arms the...
-
The Mariana snailfish (see the photo) holds the record for the world's deepest living fish. The snailfish has been found in the Mariana Trench at a depth of 26 500 feet below the water's surface. (a)...
-
A soil sample was taken from a proposed cut area in a highway construction project and sent to a soils laboratory for a compaction test, using the Standard Proctor compaction procedure. Results of...
-
Sarah agrees to sing at Joe and Sandra's June wedding and signs a contract. In April, Sarah is invited to be the top billed singer and entertainer for Carnival Cruises. It is a 2 year contract that...
-
P = 7 kN, w = 12 KN/m and angle is 32 deg Equilibrium and support reactions for beams and frames Question 2a [25 marks] Show that the following beam is statically determinate. Determine the external...
-
Determine whether the statement is true or false. If it is true, explain why. If it is false, explain why or give an example that disproves the statement. + y? dy dx = V+ y? dx dy
-
The age-old saying for investing is "buy low and sell high," but this is easier said than done. Investors who panic about falling prices sell their investments, which in turn lowers the price and...
-
For each pair of ions, determine which ion is more stable. Use resonance forms to explain your answers. (a) (b) (c) (d) (e) CH CHCH or CH CH OCH CH CH CH-CH or CH CH CH2 CH CH,_ CH, or CH,-C N: CH2...
-
Rank the following species in order of increasing acidity. Explain your reasons for ordering them as you do. H so
-
Rank the following species in order of increasing basicity. Explain your reasons for ordering them as you do. NH, CHyO- H2O CHICO NaOH NH, HS04 411
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


