Acetals can serve as protecting groups for 1,2-diols, as well as for aldehydes and ketones. When the
Question:
Acetals can serve as protecting groups for 1,2-diols, as well as for aldehydes and ketones. When the acetal is formed from acetone plus the diol, the acetal is called an acetonide. Show the acetonides formed from these diols with acetone under acid catalysis.
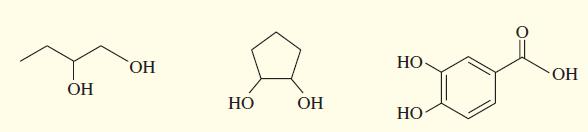
Transcribed Image Text:
HO, НО- OH OH НО OH НО
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a b c Mechanism H o...View the full answer

Answered By

Nakul Naskar
I am a organic chemist. I have experience of teaching organic chemistry to bring out Concepts about this subject which helps a student to enhance his/her knowledge and make a good impact on his educational life. Besides I can also teach Inorganic and physical chemistry.
I can monitor student performance or assist students in academic environments, such as classrooms, laboratories.
By Providing feedback to students using positive reinforcement techniques to encourage, motivate, or build confidence in students.
Prepare lesson plans or learning modules for tutoring sessions according to students' needs and goals.
During my teaching life I Maintain records of students' assessment results, progress, feedback, or school performance, ensuring confidentiality of all records.
I can clear your all doubts on chemistry by providing easy explanation.
Thank you
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Acetal formation is a characteristic reaction of aldehydes and ketones, but not of carboxylic acids. Show how you could advantageously use a cyclic acetal protecting group in the following synthesis:
-
Acetal formation is a characteristic reaction of aldehydes and ketones, but not of carboxylic acids. Show how you could advantageously use a cyclic acetal protecting group in the following synthesis:...
-
Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism: H* catalyst 2...
-
The voltage held by a voltage regulator follows a normal random variable with a mean that equals 200 volts and a standard deviation that equals 5 volts. A regulator meets the specifications if the...
-
Use electron dot structures to show why tetramethylammonium hydroxide, (CH3)4N+OH-, is an ionic compound. That is, show why hydroxide is not covalently bound to the rest of the molecule.
-
Market Model versus APT What are the differences between a k -factor model and the market model?
-
Allergy Drug A pharmaceutical company wants to test the effectiveness of a new allergy drug. The company identifies 250 females 3035 years old who suffer from severe allergies. The subjects are...
-
Willard Company manufactures three different sizes of automobile sunscreens: large, medium, and small. Willard expects to incur $360,000 of overhead costs during the next fiscal year. Other budget...
-
In the mixed cost formula Y=a+bX, what does the " Y " represent? A, total mixed cost B. fixed cost C. variable cost per unit D. activity
-
The Harvey City Comprehensive Case consists of the last problem in each chapter from Chapters 4 through 15. Completing this case essentially requires that you account for all the transactions of a...
-
Rank the following carbonyl compounds in order of increasing equilibrium constant for hydration: CH,COCH,CI CICH,CHO CH,0 CH3COCH3 CH3CHO
-
Sketch the expected proton NMR spectrum of 3,3-dimethylbutanal.
-
What is the difference between structured and unstructured data? What are some examples of each?
-
For the data in Problem 42, how would you predict demand for medical kits using (a) moving averages and (b) exponential smoothing (with alpha values equal to 0.5 and greater) for the 21st week? Data...
-
For a light ray that crosses the interface between medium 1 having index of refraction \(n_{1}\) and medium 2 having index of refraction \(n_{2}\), what relationship between \(\theta_{1}\) and...
-
The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon dioxide). The carbon dioxide on Venus might be in equilibrium with carbonate ions in minerals on...
-
Seniority quantum numbers typically measure how many fermions are in some sense "not paired" with another fermion. For the quasispin model of Problem 31.3 , define the Racah seniority $v$ through...
-
(a) Place a perfectly conducting sphere with radius a in a uniform electric field E 0 and let an origin centered electric dipole field represent the field produced by the sphere. Use this information...
-
At time t = 0, the arm is rotating about the fixed z-axis with an angular velocity = 200 rad/s in the direction shown. At that time, a constant angular deceleration begins and the arm comes to a...
-
A 2500-lbm car moving at 15 mi/h is accelerated at a constant rate of 15 ft/s 2 up to a speed of 50 mi/h. Calculate force and total time required?
-
Arrange the following compounds in order of increasing reactivity toward HNO3 in H2SO4. (The references to equations will assist you with nomenclature.) (a) Chlorobenzene, benzene, nitrobenzene (b)...
-
Rank the following compounds in order of increasing reactivity in bromination. In each case, indicate whether the principal monobromination products will be the ortho and para isomers or the meta...
-
Rank the following compounds in order of increasing reactivity in bromination. In each case, indicate whether the principal monobromination products will be the ortho and para isomers or the meta...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


