Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield
Question:
Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:
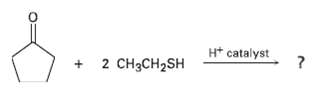
Transcribed Image Text:
H* catalyst 2 CH3CH2SH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
The same series of steps used to form an acetal is followed in thi...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the product of the following reaction. M 1) LIAID, 2) H20 Me
-
Predict the product of the following reaction. Br NaOCH3, heat -NO2
-
Predict the product of the reaction if CH3-S-CH2CH2CH2CH2-Br is heated in a polar organic solvent, such as methanol. Similarly, what would be the product for hexyl bromide in methanol? (In hexyl...
-
1. Review the six (6) goals of performance based acquisition(s); choose two and discuss how your choices can be implemented for effective management of contracts, using an actual or theoretical...
-
1. What factors of group cohesiveness were present in this team? 2. What should Joe do now to ensure the team's effectiveness and ultimate success? Use the Model of Team Effectiveness (PPT 15-9) to...
-
If two electrons are each 1.50 x 10 -10 m from a proton, as shown in Fig. E 21.37, find the magnitude and direction of the net electric force they will exert on the proton. Figure E21.37 65.0
-
What are the two leading stock exchanges in the United States today? AppendixLO1
-
Alternative allocation bases for a professional services firm. The Wolfson Group (WO) provides tax advice to multinational firms. WG charges clients for (a) direct professional time (at an hourly...
-
Find the yield to maturity on a two-year, 8% annual coupon bond with a face value of $1000. You look at the yield curve and find a one-year spot rate r1 of 2% and a two-year spot rate r2 of 5%....
-
Barton and Sons, Inc. is a small, privately-held corporation that operates two retail stores in western Kentucky. Jorge Barton and his two sons own all of the companys stock and manage the store...
-
6-Methyl-5-hepten-2-one is a constituent of lemongrass oil. How could you synthesize this substance from methyl4-oxopentanoate? CH3CH2CH2OCH3 Methyl 4-oxopentanoate
-
Ketones react with dimethyl-sulfonium methylide to yield epoxides. Suggest a mechanism (or the reaction. HICHJ)2 DMSO solvent (CH3)2S Dimethylsulfonium methylide
-
Use the Internet to identify a country with high inflation over the past year and another country that has had low inflation. For the two countries, nd the rate of money growth and the current level...
-
Low Desert Pottery works makes a variety of pottery products that it sells to retailers. The company uses a job-order costing system in which departmental predetermined overhead rates are used to...
-
ASSESSMENT CPCCBC5002A Monitor costing systems on medium rise building and construction projects Please provide answer to Part 2 - Monitor expenditure for a medium-rise project as per below...
-
Questions 6-8 refer to the same problem A sinusoidal wave with wavelength 2 m and amplitude 5 mm is traveling along the x axis. The wave is traveling in the -x direction at a speed of 2m/s At t = Os,...
-
Consider a circuit where one or more capacitors is discharged through a light bulb filament with a resistance of 3.0 0.3 . Assume that the resistance of the filament is constant (to within the stated...
-
3. For a vibrating string of length with fixed ends, each mode of vibration can be written as where wk ux(x, t) = M* sin(wxt + k) sin(x) and Mk, Ok are determined by initial conditions. For all k >...
-
The primary reason that a bank would maintain a separate compliance function is to: (a) Better manage perceived high risks. (b) Strengthen controls over the banks investments. (c) Ensure the...
-
Which of the following raises the credibility of areport? Which of the following raises the credibility of a report? Multiple Choice avoiding predictions avoiding the use of cause-effect statements...
-
Explain why it is not necessary to find the Lewis structure with the smallest formal charges to make a successful prediction of molecular geometry in the VSEPR theory. For example, write Lewis...
-
Write the mechanistic steps in the cyclization of ethyl phenylacetoacetate (ethyl 3-oxo-4-phenylbutanoate) in concentrated sulfuric acid to form naphthoresorcinol (1,3-naphthalenediol).
-
When an aldehyde or a ketone is condensed with ethyl a-chloroacetate in the presence of sodium ethoxide, the product is an α,β-epoxy ester called a glycidic ester. The...
-
The Perkin condensation is an aldol-type condensation in which an aromatic aldehyde (ArCHO) reacts with a carboxylic acid anhydride, (RCH2CO)2O, to give an a,b-unsaturated acid (ArCH "CRCO2H). The...
-
During 2024, its first year of operations, Hollis Industries recorded sales of $11,900,000 and experienced returns of $760,000. Cost of goods sold totaled $7,140,000 (60% of sales). The company...
-
What is the value of a 15% coupon bond with 11% return? Is it a discount or a premium bond?
-
A manufacturer with a December 31 taxation year end sells new machinery for $50,000 on January 2, 2022. The cost of the machinery is $20,000. The terms of the sale require an initial payment of...

Study smarter with the SolutionInn App


