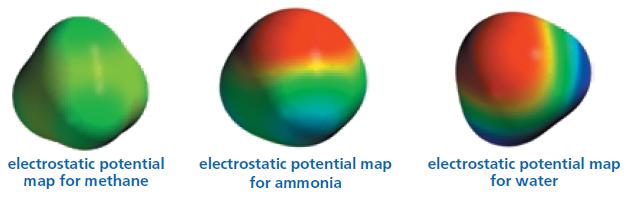
Compare the potential maps for methane, ammonia, and water. Which is the most polar molecule? Which is
Question:
Compare the potential maps for methane, ammonia, and water. Which is the most polar molecule? Which is the least polar?
Transcribed Image Text:
electrostatic potential map for methane electrostatic potential map for ammonia electrostatic potential map for water
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
Water is most polar molecule than ammonia and methane Reason The greater the electronegativity diffe...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Account for the difference in the shape and color of the potential maps for ammonia and the ammonium ion in Section 1.12.
-
After examining the potential maps for LiH, HF, and H2, answer the following questions: a. Which compounds are polar? b. Why does LiH have the largest hydrogen? c. Which compound has the most...
-
Compare the heat produced by the complete combustion of 1 mole of methane (CH4) with a mole of water gas (0.50 mole H2 and 0.50 mole CO) under the same conditions. On the basis of your answer, would...
-
just finished the business plan of his start-up company. According to the projections he carried-out, the initial investment is 1,500,000 SAR (assume that we are in the beginning of 2023), which will...
-
Nickel has two major and three minor isotopes. For the purpose of this problem, suppose that the only isotopes are 58 Ni and 60 Ni. The atomic mass of 58 Ni is 57.935 3 Da and the mass of 60Ni is...
-
Imagine you were given the position of IS manager in your organisation. What would be your approach to strategy? Think of data processing, management information systems, strategic information...
-
Redshifts of quasi-stellar objects. Astronomers call a shift in the spectrum of galaxies a redshift. A correlation between redshift level and apparent magnitude (i.e., brightness on a logarithmic...
-
Presented below is a list of balance sheet accounts in alphabetical order. Accounts payable .................. Inventories Accounts receivable ................. Land (in use) Accumulated...
-
You are considering investing in a start up company. The founder asked you for $260,000 today and you expect to get $970,000 in 14 years. Given the riskiness of the investment opportunity, your cost...
-
1. Evaluate line integrals Evaluate the line integral / 5xy ds, where C is the right half of the circle x + y = 25.
-
According to the potential map for the ammonium ion, which atom(s) is (are) most positively charged?
-
HCl is a weaker acid than HBr. Why, then, is ClCH 2 COOH a stronger acid than BrCH 2 COOH?
-
Aluminum is the primary conductor material for fabrication of microelectronic devices. Consider the composite thin film shown in the figure below. A thin film of solid aluminum is sputter-coated onto...
-
Go to: https://www.instagram.com/ryderseyewear/ on your desktop, laptop, or mobile (or a combination of all 3). You are the new Social Media Marketing Manager for Ryders Eyewear. You've been asked...
-
As leaders, it is very important that we have the ability to assess our own motivation and the motivation of others around us. It is also important to recognize the key factors involved in...
-
At the end of this exam, you will find Article 1 - " How Companies Can Prepare for a Long Run of High Inflation ". Please read the article and, when necessary, consult additional sources and the...
-
You can develop your capabilities as a manger by better understanding different ways of motivating and rewarding employees. You can also better prepare for your own career by better understanding the...
-
Topic: Project Malasakit of Kara David https://projectmalasakit.org/ What is the pros and cons of these alternative courses of the action below: Strengthen the internal organization via promoting it...
-
Use a computer algebra system to find the exact volume of the solid. Enclosed by z = 1 x 2 y 2 and z = 0
-
The registrar of a college with a population of N = 4,000 full-time students is asked by the president to conduct a survey to measure satisfaction with the quality of life on campus. The following...
-
Outline a synthesis for each of the following compounds from the indicated starting materi-als using a reaction sequence involving a diazonium salt. (a) 2-bromobenzoic acid from o-toluidine...
-
What two compounds would react in a diazo coupling reaction to form FD & C Yellow No. 6?
-
Give a curved-arrow mechanism for the electrophilic aromatic substitution reaction shown in Eq. 23.52.
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


