Convert the following line-bond structures into molecular formulas: (a) (b) (c) . CHOH . C
Question:
Convert the following line-bond structures into molecular formulas:
(a)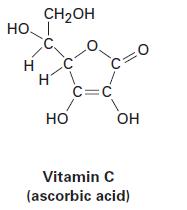
(b)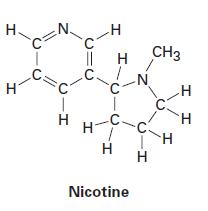
(c)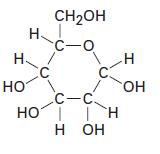
Transcribed Image Text:
НО. Н н CH₂OH С. C НО C C=C OH Vitamin C (ascorbic acid)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Convert the following hashed-wedged line formulas into condensed formulas. (a) (b) (c) in NH2 Br H-C Br Br
-
Convert the following condensed formulas into hashed-wedged line structures. (a) (b) CHCI3 (c) (CH3)2NH (d) cHuCHOCL SH ,
-
Multiple Choice Questions: 1. Which of the following is most likely a topic of discussion in macroeconomics? a. An increase in the price of a pizza b. A decrease in the production of VCRs by a...
-
Following are four independent transactions or events that relate to a local government and a voluntary health and welfare organization: 1. Made a disbursement of $25,000 from the general fund...
-
A cylinder fitted with a piston has an initial volume of 0.1 m3 and contains nitrogen at 100 kPa, 25oC. The piston is moved, compressing the nitrogen until the pressure is 1.5 MPa and the temperature...
-
Here are the comparative income statements of Delaney Corporation. DELANEY CORPORATION Comparative Income Statements For the Years Ended December 31 2017 2016 Net sales $598,000 $500,000 Cost of...
-
Five hundred kmol/h of a liquid mixture of light alcohols containing, by moles, 40% methanol (M), 35% ethanol (E), 15% isopropanol (IP), and 10% normal propanol (NP) is distilled in a sequence of two...
-
domestic firms contractually engage with manufacturers or other firms (including merchandise, or sell their products or services in foreign markets. With O Management contracting Contract...
-
Sunset Boards, Inc., is a small company that manufactures and sells surfboards in Malibu. Tad Marks, the founder of the company, is in charge of the design and sale of the surfboards, but his...
-
Show how the species in part (a) can act as Lewis bases in their reactions with HCl, and show how the species in part (b) can act as Lewis acids in their reaction with OH - . (a) CH3CHOH, (CH3)2NH,...
-
Which of the following are likely to act as Lewis acids and which as Lewis bases? Which might act both ways? (a) CH3CH2OH (d) (CH3)3B (b) (CH3)2NH (e) H3C+ (c) MgBr2 (f) (CH3)3P
-
A confidence interval for the price of gasoline from a random sample of 30 gas stations in a region gives the following statistics: y = + 4.49 s = + 0.29 a) Find a 95% confidence interval for the...
-
The air in an automobile tire with a volume of \(0.015 \mathrm{~m}^{3}\) is at \(30^{\circ} \mathrm{C}\) and \(140 \mathrm{kPa}\) (gage). Determine the amount of air that must be added to raise the...
-
Convex Productions has just received a contract to film a commercial video that will air during a major sporting event in North America, and then be available on-demand through banner advertisements...
-
The following data (and annotations) for March 2016 are for the work in process account of the first of Olympus Companys four departments used in manufacturing its nly product. Assuming that Olympus...
-
If relative volatility can be assumed constant over the change in concentration for each fraction, Eq. \((9-13)\) can be adapted to the collection of fractions from a simple binary batch...
-
(a) Design a PI controller for Problem 8.6-4(b). (b) Design a PD controller for Problem 8.6-4(c). (c) Use the results of parts (a) and (b) to repeat Problem 8.6-4(d). Problem 8.6-4(b) (c) (d) (b)...
-
Sean Astin, who played the hobbit Sam in The Lord of the Rings movies, wrote the following about an earlier film he had appeared in: "Now I was in a movie I didn't respect, making obscene amounts of...
-
Express mass density in kg/m3 and weight density in lb/ft3. 1. Find the mass density of a chunk of rock of mass 215 g that displaces a volume of 75.0 cm3 of water. 2. A block of wood is 55.9 in. x...
-
What products are obtained from the reduction of a. D-idose? b. D-sorbose?
-
What monosaccharides form the same osazone as D-sorbose?
-
What monosaccharides would be formed in a KilianiFischer synthesis starting with one of these? a. D-xylose b. L-threose
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App



