Which of the following are likely to act as Lewis acids and which as Lewis bases? Which
Question:
Which of the following are likely to act as Lewis acids and which as Lewis bases? Which might act both ways?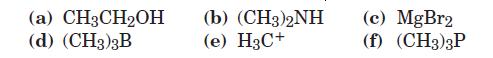
Transcribed Image Text:
(a) CH3CH2OH (d) (CH3)3B (b) (CH3)2NH (e) H3C+ (c) MgBr2 (f) (CH3)3P
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (9 reviews)
In the context of Lewis acids and bases Lewis acids are substances that can accept a pair of electrons to form a new covalent bond Lewis bases are sub...View the full answer

Answered By

Saleem Abbas
Have worked in academic writing for an a years as my part-time job.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following are Lewis acids and which are Lewis bases? a. Mg2+ b. (CH3)3C:- c. CH3NH2 d. Zn2+ e. CH3CH2OCH2CH3 f. (CH3)3C+ g. (CH3)3B h. (CH3)3N i. H:-
-
Proteins are synthesized with a particular amino acid sequence through the translation of information encoded in messenger RNA by an RNAprotein complex called a ribosome. Amino acids are specified by...
-
Show how the species in part (a) can act as Lewis bases in their reactions with HCl, and show how the species in part (b) can act as Lewis acids in their reaction with OH - . (a) CH3CHOH, (CH3)2NH,...
-
Calculate the labour turnover rate according to replacement method from the following: No. of workers on the payroll: - At the beginning of the month: 500 - At the end of the month: 600 During the...
-
Following are the adjusted current funds trial balances of Community Association for Children With Disabilities, a voluntary health and welfare organization, on June 30, 20X4: Required a. Prepare a...
-
In the problem described above, determine the entropy generated during the process if the volume of the tank is 2 m3.
-
Suppose the comparative balance sheets of Nike, Inc. are presented here. NIKE, INC. LO15 Comparative Balance Sheets May 31 ($ in millions) Assets 2017 2016 Current assets $ 9,734 $ 8,839 Property,...
-
Europa Corporation is financing an ongoing construction project. The firm will need $5,000,000 of new capital during each of the next three years. The firm has a choice of issuing new debt or equity...
-
Which of the following lessened the appeal of Investing in real estate? Multiple Choice C Hedge against inflation Professional management Financial leverage Tax Reform Act of 1986 National...
-
Matusek Corporation has been experiencing a higher than expected number of warranty claims in the current year, due mainly to less than ideal product design. For this reason, the warranty expense...
-
Convert the following line-bond structures into molecular formulas: (a) (b) (c) . CHOH . C C C=C OH Vitamin C (ascorbic acid)
-
Electrostatic potential maps of (a) acetamide and (b) methylamine are shown. Which of the two has the more basic nitrogen atom? Which of the two has the more acidic hydrogen atoms? (a) (b) Acetamide...
-
Suppose your bicycle tire is fully inflated, with an absolute pressure of 7.00 x 10 5 Pa (a gauge pressure of just under 90.0 lb/in 2 ) at a temperature of 18.0C. What is the pressure after its...
-
n1 = 15, n2 = 18, S = 280, H1: m1 > m2. Exercises 57 present sample sizes and the sum of ranks for the rank-sum test. Compute S, S, and the value of the test statistic z. Then find the P-value.
-
n1 = 25, n2 = 32, S = 850, H1: m1 m2. Exercises 57 present sample sizes and the sum of ranks for the rank-sum test. Compute S, S, and the value of the test statistic z. Then find the P-value.
-
Evaluate the matrix element $\left\langle j_{1} j_{2} J\left|T_{k q}(1) ight| j_{1}^{\prime} j_{2}^{\prime} J^{\prime} ightangle$, where the tensor operator $T_{k q}(1)$ operates only on the part of...
-
Mark Gold opened Gold Roofing Service on April 1. Transactions for April are as follows: 1 Gold contributed \(\$ 15,000\) of his personal funds in exchange for common stock to begin the business. 2...
-
n1 = 20, n2 = 30, S = 400, H1: m1 < m2. Exercises 57 present sample sizes and the sum of ranks for the rank-sum test. Compute S, S, and the value of the test statistic z. Then find the P-value.
-
An article in the Wall Street Journal discussed why the hotel workers' union in New York City was against a proposal for more hotels to be built in Midtown Manhattan: "The union is concerned that...
-
The first national bank pays a 4% interest rate compound continuously. The effective annual rate paid by the bank is __________. a. 4.16% b. 4.20% c. 4.08% d. 4.12%
-
What two monosaccharides can be degraded to a. D-arabinose? b. D-glyceraldehyde? c. L-ribose?
-
Aldohexoses A and B form the same osazone. A is oxidized by nitric acid to an optically active aldaric acid, and B is oxidized to an optically inactive aldaric acid. Ruff degradation of either A or B...
-
What kind of aldohexose would form L-glyceraldehyde when its acetal is oxidized with periodic acid?
-
The following information is provided by Garden Gears for a new product it recently introduced: Total unit cost $50 Desired ROI per unit $22 Target selling price $72 How much is Garden Gears'...
-
Solid bank loan P5 million to a borrower on January 1, 2018. The terms of the loan require principal payments of P1 million each year for five years plus interest at 8%. The first principal and...
-
3) Assuming annual sales of $250,000 and a 50% gross (contribution) margin, calculate the following a. Average collection period if ending receivables total $45,000 b. Ending days-on-hand of...

Study smarter with the SolutionInn App


