Electrostatic potential maps of (a) acetamide and (b) methylamine are shown. Which of the two has the
Question:
Electrostatic potential maps of (a) acetamide and (b) methylamine are shown. Which of the two has the more basic nitrogen atom? Which of the two has the more acidic hydrogen atoms?
(a)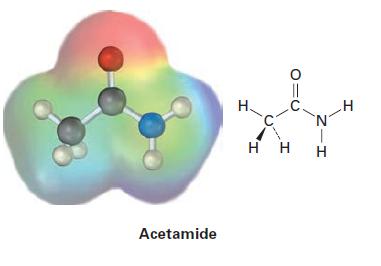
(b)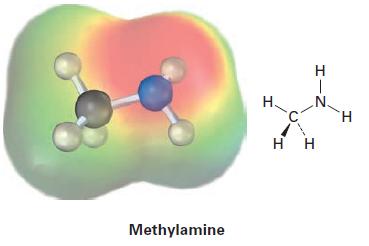
Transcribed Image Text:
Acetamide H. 010 HH Z-H Н H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Acid According to BronstedLowry acid is the substance which donate hydrogen ion According Lewis subs...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The difference in positive-charge distribution in an amide that accepts a proton on its oxygen or its nitrogen atom can be visualized with electrostatic potential maps. Consider the electrostatic...
-
The molecular electrostatic potential maps for LiH and HF are shown here. Does the apparent size of the hydrogen atom (shown as a white sphere) tell you whether it is an electron acceptor or an...
-
Electrostatic potential maps of a typical amide (acetamide) and an acyl azide (acetyl azide) are shown. Which of the two do you think is more reactive in nucleophilic acyl substitution reactions?...
-
True or False: 1. Economists are not completely in agreement on what constitutes money for all purposes. 2. People use nontransaction accounts primarily because they generally pay higher interest...
-
The postclosing trial balance of the general fund of Serene Hospital, a not-for-profit entity, on December 31, 20X1, was as follows: During 20X2 the following transactions occurred: 1. Provided the...
-
A 3 m3 tank initially contains air at 100 kPa and 25oC. The tank is connected to a supply line at 550 kPa and 25oC. The valve is opened, and air is allowed to enter the tank until the pressure in the...
-
Operating data for Joshua Corporation are presented below. 2017 2016 Sales revenue $800,000 $600,000 Cost of goods sold 520,000 408,000 Selling expenses 120,000 72,000 LO15 Income tax expense 30,000...
-
The mean per capita daily water consumption in a village in Bangladesh is about 83 liters per person and the standard deviation is about 11.9 liters per person. Random samples of size 50 are drawn...
-
Bond J has a coupon rate of 6 percent. Bond K has a coupon rate of 1 0 percent. Both bonds have 9 years to maturity, make semiannual payments, and have a YTM of 8 percent. If interest rates suddenly...
-
Matusek Corporation has been experiencing a higher than expected number of warranty claims in the current year, due mainly to less than ideal product design. For this reason, the warranty expense...
-
Which of the following are likely to act as Lewis acids and which as Lewis bases? Which might act both ways? (a) CH3CH2OH (d) (CH3)3B (b) (CH3)2NH (e) H3C+ (c) MgBr2 (f) (CH3)3P
-
Convert the following molecular model of aspirin into a line-bond structure, and identify the hybridization of each carbon atom (gray = C, red = O, ivory = H). Aspirin (acetylsalicylic acid)
-
Thirty samples of size 3, available in the worksheet C16P4 in the OM5 Data Workbook were taken from a machining process over a 15-hour period. Construct control charts using the Excel template x-Bar...
-
The adjusted trial balance section of Menlo Company's worksheet shows a \(\$ 1,500\) debit balance in utility expense. At the end of the accounting period the accounting manager accrues an additional...
-
Identify each of the 10 amount columns of the worksheet and indicate to which column the adjusted balance of the following accounts would be extended: a. Accounts Receivable b. Accumulated...
-
Using the data from Table 3.3, show the effect on world output if each country moved toward specialization in the production of its comparative-disadvantage good. TABLE 3.3 Comparative Advantage as a...
-
The Professional Winner was RJ Andrews from Info We Trust, for the video Are Gazelles Endangered? (a) Watch this video. What data are this video conveying? (b) You can interact with the data and...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located outside the focal length of a converging lens. (b) Is the image real or virtual? (c) Is it upright or...
-
Over time, the gap between the wages of workers with college degrees and the wages of workers without college degrees has been increasing. Shouldn't this gap have increased the incentive for workers...
-
Listed below are several terms and phrases associated with basic assumptions, broad accounting principles, and constraints. Pair each item from List A (by letter) with the item from List B that is...
-
Which amino acids in Table 23.1 have more than one asymmetric carbon? Table 23.1 The Most Common Naturally Occurring Amino Acids The amino acids are shown in the form that predominates at...
-
Why are the carboxylic acid groups of the amino acids so much more acidic (pK a ~ 2) than a carboxylic acid such as acetic acid (pK a = 4.76)?
-
A mixture of seven amino acids (glycine, glutamate, leucine, lysine, alanine, isoleucine, and aspartate) is separated by TLC. Explain why only six spots show up when the chromatographic plate is...
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


