Convert the following molecular model of aspirin into a line-bond structure, and identify the hybridization of each
Question:
Convert the following molecular model of aspirin into a line-bond structure, and identify the hybridization of each carbon atom (gray = C, red = O, ivory = H).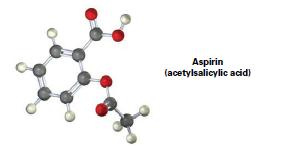
Transcribed Image Text:
Aspirin (acetylsalicylic acid)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Aspirins chemical formula is C9H8O4 and its systematic name is acetylsalicylic ...View the full answer

Answered By

User l_1013947
I possess a comprehensive understanding of programming languages such as C++, Python, HTML, CSS, and Jupyter Notebook. These technical skills enable me to develop robust software solutions and create visually appealing web pages. With my expertise in coding, I can effectively tackle complex programming tasks and deliver high-quality results.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
In the hydrocarbon (a) What is the hybridization at each carbon atom in the molecule? (b) How many Ï bonds are there in the molecule? (c) How many Ï bonds? (d) Identify all the 120o bond...
-
Convert the following molecular model of ethane, C 2 H 6 , into a structure that uses wedged, normal, and dashed lines to represent three-dimensionality. Ethane
-
A chemical is spilled into a lake of pure water and the concentration of chemicals in this lake is 4 If 20 of the water in the lake is replaced with clean in one month then. What will be the...
-
A group of civic-minded merchants in Eldora organized the Committee of 100 for establishing the Community Sports Club, a not-for-profit sports organization for local youth. Each of the committees 100...
-
A 4 kg iron block initially at 300oC is dropped into an insulated tank that contains 80 kg of water at 25oC. Assuming the water that vaporizes during this process condenses back in the tank and the...
-
Here is fi nancial information for Glitter Inc. December 31, 2017 December 31, 2016 Current assets $106,000 $ 90,000 Plant assets (net) 400,000 350,000 LO15 Current liabilities 99,000 65,000...
-
The so-called Internet Bubble gripped stock markets in 1998, 1999, and 2000, as discussed in the chapter. Internet stocks traded at multiples of earnings and sales rarely seen in stock markets....
-
You have invested 20% of your money in Microsoft, 45% in GM, and the rest in Walmart. Walmart has an expected return of 14%, and the expected returns of Microsoft and GM are 12.65% and 3.12%,...
-
On January 1, 2018, Jana started a small flower merchandising business that she named Jana's Flowers. The company experienced the following events during the first year of operation: 1. Started the...
-
Electrostatic potential maps of (a) acetamide and (b) methylamine are shown. Which of the two has the more basic nitrogen atom? Which of the two has the more acidic hydrogen atoms? (a) (b) Acetamide...
-
Is either of the following reactions likely to take place according to the pKa data in Table 1.2? (a) (b) HCN + CH3CO- Na+ Na+ CN + CH3COH
-
Describe each stage of the product life cycle. Select a representative product or service that exemplifies each of the stages.
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a converging lens, closer to the lens than to the focal point. (b) Is the image...
-
Power efficiency has become very important for modern processors, particularly for embedded systems. Create a version of gcc for two architectures that you have access to, such as x86, RISC-V,...
-
There is a movement toward wireless mobile computing using thin-client technology. Go to the Web and visit some of the ma jor computer vendors that are producing thin-client products such as handheld...
-
Draw a B-tree of order 4 and height 3 containing the fewest elements. Show an example of a split that would be applied by inserting the fewest number of elements.
-
Repeat Example 10-4, except calculate the diameter at the bottom of the column. Example 10-4 A distillation column is separating n-hexane from n-heptane using 1-in. ceramic Intalox saddles. The...
-
If the labor supply curve shifts to the left and the labor demand curve remains unchanged, what will happen to the equilibrium wage and the equilibrium level of employment? Illustrate your answer...
-
Interest Compounded Annually. When P dollars is invested at interest rate i, compounded annually, for t years, the investment grows to A dollars, where A = P(1 + i) t . Trevor's parents deposit $7800...
-
a. How many different octapeptides can be made from the 20 naturally occurring amino acids? b. How many different proteins containing 100 amino acids can be made from the 20 naturally occurring amino...
-
Suppose you are trying to synthesize the dipeptide Val-Ser. Compare the product that would be obtained if the carboxyl group of N-protected valine were activated with thionyl chloride with the...
-
In determining the primary structure of insulin, what would lead you to conclude that it had more than one polypeptide chain?
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


