Convert the following molecular model of ethane, C 2 H 6 , into a structure that uses
Question:
Convert the following molecular model of ethane, C2H6, into a structure that uses wedged, normal, and dashed lines to represent three-dimensionality.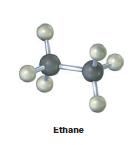
Transcribed Image Text:
Ethane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Threedimensional geometry To represent the threedimensional geometry of a molecule we need to use a ...View the full answer

Answered By

User l_1013947
I possess a comprehensive understanding of programming languages such as C++, Python, HTML, CSS, and Jupyter Notebook. These technical skills enable me to develop robust software solutions and create visually appealing web pages. With my expertise in coding, I can effectively tackle complex programming tasks and deliver high-quality results.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Convert the following representation of ethane, C2H6. Into a conventional drawing that uses solid, wedged, and dashed lines to indicate tetrahedral geometry around each carbon (gray = c, ivory =H).
-
Convert the following molecular model of aspirin into a line-bond structure, and identify the hybridization of each carbon atom (gray = C, red = O, ivory = H). Aspirin (acetylsalicylic acid)
-
Assign R or S configuration to the chirality center in the following molecular model of the amino acid methionine (blue = N, yellow =S):
-
Why is an increase in price more likely to decrease the total revenue of a seller in the long run than in the short run?
-
Brookdale Hospital hired an inexperienced controller early in 20X4. Near the end of 20X4, the board of directors decided to conduct a major fund-raising campaign. They wished to have the December 31,...
-
A heat engine receives heat from a source at 2000 K at a rate of 500 kW and rejects the waste heat to the atmosphere at 300 K. The net output from the engine is 300 kW. Determine (a) the reversible...
-
Summary fi nancial information for Gandaulf Company is as follows. Dec. 31, 2017 Dec. 31, 2016 LO15 Current assets $ 200,000 $ 220,000 Plant assets 1,040,000 780,000 Total assets $1,240,000...
-
Determine the number of salespeople FireSpot needs if it has 1,000 retail customer accounts that need to be called on five times per year. Each sales call lasts approximately 2.5 hours, and each...
-
The Wei Corporation expects next year's net income to be $15 million. The firm is currently financed with 45% debt. Wei has $14 million of profitable investment opportunities, and it wishes to...
-
Consider the following selected amounts and account balances of Lords: Show how this company reports current assets on the balance sheet. Not all data are used. Is Lords a service company, a...
-
Electrostatic potential maps of (a) acetamide and (b) methylamine are shown. Which of the two has the more basic nitrogen atom? Which of the two has the more acidic hydrogen atoms? (a) (b) Acetamide...
-
Is either of the following reactions likely to take place according to the pKa data in Table 1.2? (a) (b) HCN + CH3CO- Na+ Na+ CN + CH3COH
-
A market has the following limit orders standing on its book for a particular stock: Ian submits a day order to sell 1,000 shares, limit d19.83. Assuming that no more buy orders are submitted on that...
-
Prove that Eq. (19.34) gives the simplest multi-gluon and gluon-quark states that contain an \(\mathrm{SU}(3)\) color singlet in the decomposition. Data from Eq. 19.34 (GG)1: (88)1 (Gqq) : [8 (383)8]...
-
In question 70, what is the probability that of the 100 cars test-driven, more than 35 cars get more than 45 miles per gallon? How many of the 100 cars tested would you expect to get more than 45...
-
Construct the braid group products (a) (b) using the algorithm of Fig. 29.16 . Data from Fig. 29.16
-
Worksheet The adjusted trial balance columns of a worksheet for Bond Corporation are shown below. The worksheet is prepared for the year ended December 31. Complete the worksheet by (a) entering the...
-
The Healthy Catering Service had the following transactions in July, its first month of operations: 1 Kelly Foster contributed \(\$ 18,000\) of personal funds to the business in exchange for common...
-
If the labor demand curve shifts to the left and the labor supply curve remains unchanged, what will happen to the equilibrium wage and the equilibrium level of employment? Illustrate your answer...
-
Which of the following statements is false? a. Capital leases are not commonly reported in a Capital Projects Fund. b. A governmental entity may report a Capital Project Fund in one year but not the...
-
Determine the amino acid sequence of a polypeptide from the following results: Acid hydrolysis gives Ala, Arg, His, 2 Lys, Leu, 2 Met, Pro, 2 Ser, Thr, Val. Carboxypeptidase A releases Val. Edmans...
-
How would a protein that resides in the interior of a membrane fold, compared with the water-soluble protein just discussed?
-
Which of the following parameters would be different for a reaction carried out in the presence of a catalyst, compared with the same reaction carried out in the absence of a catalyst? G , , , AS", ,...
-
A person purchased a $181,873 home 10 years ago by paying 20% down and signing a 30-year mortgage at 8.4% compounded monthly. Interest rates have dropped and the owner wants to refinance the unpaid...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
NEED THIS EXCEL TABLE ASAP PLEASE!!!! Presupuesto Operacional y C lculo del COGS Ventas Proyectadas: Ventas Proyectadas: $ 4 5 0 , 0 0 0 Precio por unidad: $ 4 5 0 Unidades vendidas: 4 5 0 , 0 0 0 4...

Study smarter with the SolutionInn App


