Draw and name the products you would expect to obtain by reaction of the following substances with
Question:
Draw and name the products you would expect to obtain by reaction of the following substances with Cl2 and FeCl3 (blue=N, reddish brown=Br):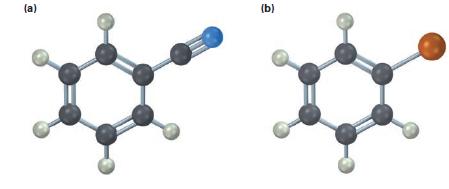
Transcribed Image Text:
(a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Our aim here is to draw and name the compounds represented by given molecular models We also have to draw and name products obtained by the reaction o...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the reagents represented by the letters a?c in the following scheme: Br Br Br Br
-
Show the products you would expect to obtain from reaction of glyceryl trioleate with the following reagents: (a) Excess Br2 in CH2Cl2, (b) H2/Pd (c) NaOH/H2C (d) O3, then Zn/CH3CO2H (e) LiAlH4, then...
-
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis). (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (m) (n) (o) (CH2 = CH)2 CuLi + CH3CH2CH= CHCH2Br...
-
Sandra, a sole proprietor, is an interior designer and has thefollowing account balances on December 31, 20X0: Cash $2,500,Inventory $9,000, Equipment $25,000, Accumulated depreciation$5,000, Liabi 2...
-
Consider the following incomplete table of a merchandisers profit data: Requirement 1. Complete the table by computing the missingamounts. Net Sales Gross Sales Discounts 3,300 2,900 Profit 28,700...
-
Discuss Implication of TQM on organization Discuss Foundation of Quality Management What are the Utility of quality awards Discuss quality awards of other nation Discuss Malcom Baldrige Award Discuss...
-
What is chance variation? What are the causes of it? How can it be altered? LO.1
-
1. An eight-month investigation by OFHEO concluded that slack standards at Fannie Mae created a corporate culture that emphasized stable earnings at the expense of accurate financial disclosures....
-
Houston Dialysis Center is a department of Houston General Hospital, a full-service, not-for-profit acute care hospital with 325 beds. The bulk of the hospitals facilities are devoted to inpatient...
-
There are three resonance structures of naphthalene, of which only one is shown. Draw the other two. Naphthalene
-
Line-bond structures appear to imply that there are two different isomers of 1,2-dibromobenzene, one with the bromine bearing carbon atoms joined by a double bond and one with the bromine-bearing...
-
Suppose that a mutual fund has a five-year nominal rate of return of 50 percent. If this five-year rate was constructed from semiannual effective rates, what would be the equivalent EAR? a. 9.10% b....
-
Show that the scalar $K$, which, according to Eq. (5.366), is constructed from the extrinsic curvature as $K=g^{\mu v} K_{\mu u}$, is equal to the covariant divergence of the normal vector field,...
-
An aircraft is in flight, and its \(\mathrm{TAS}=220 \mathrm{~m} / \mathrm{s}\). The ambient temperature is \(T=253 \mathrm{~K}\). What is the stagnation temperature on its leading edge?
-
A diver's watch resists an absolute pressure of 5.5 bar. At an ocean having density of \(1025 \mathrm{~kg} / \mathrm{m}^{3}\) and exposing an atmospheric pressure of \(1 \mathrm{bar}\), what depth...
-
Estimate TAS if an aircraft is at ALT \(=9500 \mathrm{~m}\) and its Mach number \(M\) is 0.5 .
-
The Mach number of an aircraft is \(M=0.9\), and the local temperature is \(T=-10^{\circ} \mathrm{C}\). What is its airspeed?
-
Critics of Nike often complain that its shoes cost almost nothing to make, yet they are priced so high. Identify the elements of providing and communicating value that add to Nike's cost structure...
-
In Exercises 15 through 30, find the derivative dy/dx. In some of these problems, you may need to use implicit differentiation or logarithmic differentiation. y ex + et -2x 1 + e
-
The N-F bond is more polar than the N-H bond, but NF 3 has a smaller dipole moment than NH 3 . Explain this curious result. NH 3 .... NF 3 = 1.5D = 0.2D
-
For each of the following compounds: 1. Draw the Lewis structure. 2. Show how the bond dipole moments (and those of any nonbonding pairs of electrons) contribute to the molecular dipole moment. 3....
-
Classify the following hydrocarbons, and draw a Lewis structure for each one. A compound may fit into more than one of the following classifications: (a) (CH 3 CH 2 ) 2 CHCH(CH 3 ) 2 (b) CH 3 CHCHCH...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...

Study smarter with the SolutionInn App


