There are three resonance structures of naphthalene, of which only one is shown. Draw the other two.
Question:
There are three resonance structures of naphthalene, of which only one is shown. Draw the other two.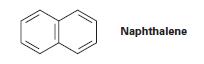
Transcribed Image Text:
Naphthalene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
ic aromatic hydrocarbon consisting of two benzene rings fused toge...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Look at the three resonance structures of naphthalene shown in Section 15.7, and account for the fact that not all carboncarbon bonds have the same length. The C1C2 bond is 136 pm long, whereas the...
-
The organic molecules shown here are derivatives of benzene in which six-member rings are "fused" at the edges of the hexagons. (a) Determine the empirical formula of benzene and of these three...
-
Draw three resonance structures of sulfur dioxide (SO2). Indicate the most plausible structure(s).
-
. 4. Bank overdrafts repayable on * 1 point demand may be included in the cash and cash equivalent balance. True O False
-
The account balances for Pauls Furniture, Inc., for the year ended August 31, 2012, are presented next in random order: Requirements 1. Prepare Pauls Furnitures single-step income statement. 2. Would...
-
B. To address Brittney's concern about her superannuation balance, discuss the concept of Salary Sacrifice to super and how carry-forward (catch-up) super contributions work. Her employer contributes...
-
Name and describe each of the costs of quality. What is the best way to reduce the costs of quality? LO.1
-
Why are convertible bonds less risky than stock but usually more risky than nonconvertible bonds?
-
John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter, Samantha. In 2021, John worked as a computer technician at a local university earning a salary of $152,200, and...
-
Show the structure of Teflon by drawing several repeating units. The monomer unit is tetrafluoroethylene, F 2 C=CF 2 .
-
Draw and name the products you would expect to obtain by reaction of the following substances with Cl 2 and FeCl 3 (blue=N, reddish brown=Br): (a) (b)
-
How does the Federal unified transfer tax differ from an income tax?
-
Air at \(1 \mathrm{~atm}\) and \(25^{\circ} \mathrm{C}\) flows in a 4-cm-diameter glass pipe at a velocity of \(7 \mathrm{~m} / \mathrm{s}\). The friction factor for this flow is (a) 0.0266 (b)...
-
Find the regime of the boundary layer at the location of \(0.1 \mathrm{~m}\) from the leading edge of an aerofoil, if the airspeed is \(80 \mathrm{~ms}^{-1}\), and air condition is at that of sea...
-
``Nuclear Deterrence,'' by Harvey A . Smith, UMAP327. The author analyzes the stability of the arms race, assuming objectives similar to those suggested by General Taylor. The module develops...
-
A saturated vapor feed at \(1000.0 \mathrm{kmol} / \mathrm{h}\) of methanol \((5.0 \mathrm{~mol} \%)\) and water \((95.0 \mathrm{~mol} \%)\) is fed to a distillation column with 18 stages plus a...
-
Use the partition functions in Equations 1.98, 1.99, and 1.100 to find the translational, rotational, and vibrational contributions to the average energy of a diatomic molecule. Compare each result...
-
How strategic planning is carried out at the corporate and divisional levels? How strategic planning is carried out at the business unit level?
-
Write each fraction as a percent. 7 50
-
Which of the following compounds show cis-trans isomerism? Draw the cis and trans isomers of those that do. a. CHF=CHF b. F 2 C=CH 2 c. CH 2 =CH-CH 2 -CH 3 d. e. f. CHCH,
-
Give the relationship between the following pairs of structures. The possible relationships are: same compound cis-trans isomers constitutional isomers (structural isomers) not isomers (different...
-
The C=O double bond has a dipole moment of about 2.4 D and a bond length of about 1.23 . (a) Calculate the amount of charge separation in this bond. (b) Use this information to evaluate the relative...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


