Which of the following compounds show cis-trans isomerism? Draw the cis and trans isomers of those that
Question:
Which of the following compounds show cis-trans isomerism? Draw the cis and trans isomers of those that do.
a. CHF=CHF
b. F2C=CH2
c. CH2=CH-CH2-CH3
d.
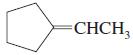
e.
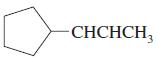
f.
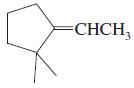
Transcribed Image Text:
CHCH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
The cis trans isomerism is shown by the alkenes only The essenti...View the full answer

Answered By

Mr. VISHAL
I'm pursuing msc. Chemistry (organic specialisation). I have deep knowledge of chemistry and i can explain the concepts in a very simple way. I also like to give lectures on specific topics.
My linkedin profile is:
www.linkedin.com/in/vishal-chnaliya-66a85514b
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds are chiral? Draw each compound in its most symmetric conformation, star (*) any asymmetric carbon atoms, and draw any mirror planes. Label any meso compounds. You may...
-
Which of the following compounds show only a single peak in their 1H NMR spectrum? a. CH3CH2OCH2CH3 b. c. CH,CH,CCI
-
Which of the following compounds are aromatic? a. b. c. Cycloheptatrienyl cation d. e. f. g. Cyclononatetraenyl anion h. CH2=CHCH=CHCH=CH2
-
In Exercises find the positive values of p for which the series converges. n=1 n
-
BH+ ClO-4 is a salt formed from the base B (Kb = 1.00 10-4) and perchloric acid. It dissociates into BH+, a weak acid, and ClO-4, which is neither an acid nor a base. Find the pH of 0.100 M BH+ClO-4.
-
Was your team composed mostly of people you have worked with previously in teams? If so, do you think the discussion was more effective or less effective than when making decisions with people who...
-
Explain why stepwise regression is used. What is its value in the model-building process?
-
Effect of temporary differences on income taxes. Woodward Corporation purchases a new machine for $50,000 on January 1, 2008. The machine has a four-year estimated service life and an estimated...
-
P6.5 (LO 2, 4) (Analysis of Alternatives) Julia Baker died, leaving to her husband Brent an insur- ance policy contract that provides that the beneficiary (Brent) can choose any one of the following...
-
The iceberg effect in organizations is the underlying factors and circumstances which are not visible and have organizational implications due to strong undercurrents. When combined with cliquism, it...
-
Two compounds with the formula CH 3 -CH=N-CH 3 are known. (a) Draw a Lewis structure for this molecule, and label the hybridization of each carbon and nitrogen atom. (b) What two compounds have this...
-
Give the relationship between the following pairs of structures. The possible relationships are: same compound cis-trans isomers constitutional isomers (structural isomers) not isomers (different...
-
ICU Window, Inc., is trying to determine its cost of debt. The firm has a debt issue outstanding with seven years to maturity that is quoted at 93 percent of face value. The issue makes semiannual...
-
Pulleys C and D in Figure are fastened together. Weights A and B are supported by ropes wound around the pulleys as shown. The radius for pulley C is 187 mm and the radius for pulley D is 138 mm. If...
-
As a leader what are some of the thoughtful and creative ideas that you have implemented to motivate your team and increase job satisfaction?
-
A Chinese smartphone maker TECNO Ltd has provided you with a summary of its price and cost information for one of its product segments (tablets). It is based on 2018 income statement. Units produced...
-
The following projected financial data is available for the single product of Janis Ltd:- October November December Sales (unit) 50,000 65,000 65,000 Production (unit) 70,000 60,000 50,000 Opening...
-
I would appreciate freehand sketches for the top, side views, and front views. D C 6 50 B 2.75 A a 5 4 3 2 1 .45 UNLESS OTHERWISE SPECIFIED: DIMENSIONS ARE IN MILLIMETERS SURFACE FINISH: TOLERANCES:...
-
The 9-m steel beam is being hoisted from its horizontal position by the two cables attached at A and B. If the initial angular accelerations of the hoisting drums are a 1 = 0.5 rad/s 2 and a 2 = 0.2...
-
Show that the block upper triangular matrix A in Example 5 is invertible if and only if both A 11 and A 22 are invertible. Data from in Example 5 EXAMPLE 5 A matrix of the form A = [ A11 A12 0 A22 is...
-
What products are formed when gach of the following ethers reacts with concenffated aqueous HI? 2-ethoxy-2,3-dimethylbutane
-
From what epoxide and what nucleophile colld each of the following compound be prepared Inppued? (Assums each is racemic.) C,H OH/H,O CH CH2 sodium azide
-
Suggest a Williamron other cynthosis, if one is possible, for each of the following compounds. If no Williamson ether synthesis is possible, explain why. (CH3)2CH---S---CH3
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


