How would you prepare benzylamine, C 6 H 5 CH 2 NH 2 , from each of
Question:
How would you prepare benzylamine, C6H5CH2NH2, from each of the following starting materials?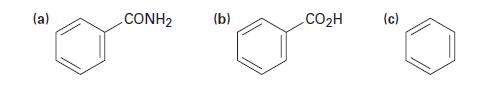
Transcribed Image Text:
(a) CONH2 (b) CO₂H (c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To prepare benzylamine C6H5CH2NH2 from each of the given starting materials we need to perform speci...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show how you might prepare benzylamine from each of the following compounds: (a) (b) (c) Benzyl bromide (two ways) (d) Benzyl tosylate (e) Benzaldehyde (f) Phenylnitromethane (g) NH2 Benzylamine CN...
-
How would you prepare the following compounds from the given starting materials? a. b. c. d. CH3CH2CH CH3CHCH N(CH3)2 CH CHCH OCH3
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
In Exercises 6567, consider a scalar function and a vector field F in space. Determine whether the expression is a vector field, a scalar function, or neither. Explain. div[curl()]
-
List and describe four important differences between managerial and financial accounting.
-
Convert the integral from rectangular coordinates to both cylindrical and spherical coordinates, and evaluate the simplest iterated integral. a-x IIZI /a-x ra+ a-x-y -a x dz dy dx
-
The minimum wage. The federal government sets the minimum hourly wage that employers can pay a worker. Labor wants a high minimum wage, but many economists argue that too high a minimum makes...
-
1. What forces for change are evident at the Oconomo plant? 2. What is the primary type of change neededchanging things or changing the people and culture? Can the Wisconsin plant be saved by...
-
AL Mustapha Company produces different products through automation. The company incurs largeamount of overheads in the factory on power alone , that is semi-variable in nature. The details of power...
-
Which compound is more basic, triethylamine or aniline? Does the following reaction proceed as written? (CH3CH)2NH+ CI + NH NH3+ CI + (CH3CH2)2N
-
How might you prepare the following amines from 1-bromobutane? (a) Butylamine (b) Dibutyl amine (c) Pentylamine
-
Backwoods Mining Co. acquired mineral rights for $53,200,000. The mineral deposit is estimated at 19,000,000 tons. During the current year, 2,500,000 tons were mined and sold. a. Determine the amount...
-
5) A frictionless rod of length L rotates counterclockwise in the with constant angular speed w at an angle a to the z axis. A bead of mass m, free to slide on the rod, leaves the origin with initial...
-
1) Louisa is a corn farmer in Illinois. She anticipates a harvest in August of 3 million bushels of yellow corn. Today is May. Louise plans to hedge her sale of corn in August using corn futures...
-
2. DETAILS MY NOTES In a statistical test, we have a choice of a left-tailed test, a right-tailed test, or a two-tailed test. Is it the null hypothesis or the alternate hypothesis that determines...
-
2. The model of a two-story building shown in Figure 2. The girders are assumed to be rigid, and the columns have flexural rigidities EI and EI2, with negligible masses. The stiffness of each column...
-
Prepare journal entries to record these transactions. (List all debit entries before credit entries. Credit account titles are automatically indented when amount is entered. Do not indent manually....
-
Under a welfare plan, poor people are given a lump-sum payment of L. If they accept this welfare payment, they must pay a high marginal tax rate, = 1/2, on anything they earn. If they do not accept...
-
Provide a few individual examples who revealed what aspects of emotional intelligence?
-
Propose a plausible synthesis for each of the following transformations: a. b. c. Br Br
-
There are four constitutional isomers with molecular formula C 3 H 9 N. Draw a Lewis structure for each isomer and determine the number of lone pairs on the nitrogen atom in each case?
-
Would water be a suitable proton source to protonate the following compound? ONa
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


