Which compound is more basic, triethylamine or aniline? Does the following reaction proceed as written? (CH3CH)2NH+ CI
Question:
Which compound is more basic, triethylamine or aniline? Does the following reaction proceed as written?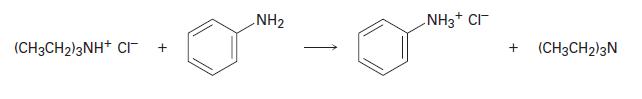
Transcribed Image Text:
(CH3CH₂)2NH+ CI + NH₂ NH3+ CI + (CH3CH2)2N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
Triethylamine NEt3 is more basic than aniline C6H5NH2 The basicity of a compound depends on its abil...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which compound is more reactive toward electrophilic substitution (for example, nitration)? OCH or b. CH,CH3 a. ETor
-
Which compound is more likely to be carcinogenic? b. or
-
Which compound is more likely to be a general anesthetic? CH3CH2CH2OH or CH3OCH2CH3
-
Write a formula for a function g whose graph is similar to f(x) but satisfies the given conditions. Do not simplify the formula. f(x) = 3x 3x + 2 (a) Shifted right 2000 units and upward 70 units (b)...
-
Distinguish between line and staff positions. Give two examples of each in a university setting.
-
Consider a circular lawn with a radius of 10 feet, as shown in the figure. Assume that a sprinkler distributes water in a radial fashion according to the formula (measured in cubic feet of water per...
-
Paying for Harvard. Harvard charged $5900 for tuition, room, and board in 1976. The 2007 charge was $42,078. Express Harvards 1976 charges in 2007 dollars. Did the cost of going to Harvard go up...
-
Rick and Stacy Stark, a married couple, are interested in purchasing their first boat. They have decided to borrow the boats purchase price of $100,000. The family is in the 28% federal income tax...
-
What is the portfolio weight for stock A for a portfolio that has 140 shares of Stock A that sell for $52.46 per share and 131 shares of Stock B that sell for $73.64 per share? Report as a % with 2...
-
Furan, the oxygen-containing analog of pyrrole, is aromatic in the same way that pyrrole is. Draw an orbital picture of furan, and show how it has six electrons in its cyclic conjugated orbitals....
-
How would you prepare benzylamine, C 6 H 5 CH 2 NH 2 , from each of the following starting materials? (a) CONH2 (b) COH (c)
-
What is meant by operating cost unit?
-
A company must decide between scrapping or reworking units that do not pass inspection. The company has 16,000 defective units that have already cost $132,000 to manufacture. The units can be sold as...
-
according to the phase rule, the triple point of a pure substance is A. invariant B. u nivariant C. bivariant D. none of the above
-
33. If the equipment in the previous question had sold for $15,000, the correct entry would be: a. Cash debit $15,000. Gain credit $3,000. $12,000 Equipment credit b. Cash debit $15,000. Debit a loss...
-
The banks play a central role in financial intermediation in New Zealand. 1.What is financial intermediation? Who performs it? and why is it important? 2.What is Qualitative Asset transformation...
-
Consider the following information attributed to the material management department Budgeted usage of materials - handling labor - hours 3,700 Budgeted cost pools: Fixed costs $166,500 Variable costs...
-
Prescott (2004) argued that U.S. employees work 50% more than do German, French, and Italian employees because European employees face lower marginal tax rates. Assuming that workers in all four...
-
How do individual companies respond to economic forces throughout the globe? One way to explore this is to see how well rates of return for stock of individual companies can be explained by stock...
-
Draw a Lewis structure for each of the following compounds: (a) C 2 H 6 (b) C 2 H 4 (c) C 2 H 2 (d) C 3 H 8 (e) C 3 H 6 (f) CH 3 OH
-
Identify reagents that can be used to achieve each of the following transformations: a. b. c. Br
-
Borane (BH 3 ) is very unstable and quite reactive. Draw a Lewis structure of borane and explain the source of the instability.
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


