Identify AN: 1. CH3CCI AlCl3 2. H20 ROOH A HCI C+D H20 Br2 Soclz E+F CH3I 1.
Question:
Identify A–N:
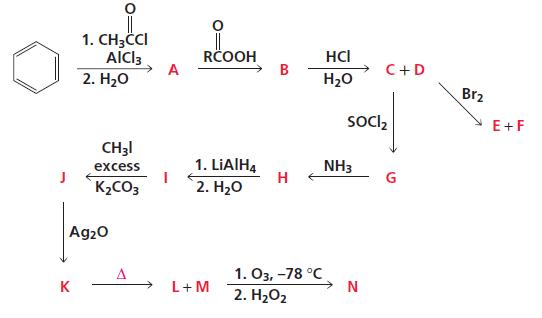
Transcribed Image Text:
1. CH3CCI AlCl3 2. H20 RČOOH A HCI C+D H20 Br2 Soclz E+F CH3I 1. LIAIHĄ H 2. H20 excess NH3 G K2CO3 Ag20 1. Оз, -78°C K L+M 2. H202
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify any formal charges in the following structures: a. b. c. d. -N=N:
-
Identify the major and minor products for each of the following E2 reactions: a. b. c. d. e. f. CI NaOEt
-
Identify the reagents you would use to achieve each of the following transformations: (a) (b)
-
Damages require one person to pay money as compensation for another persons loss. Damages are therefore a private matter. Since the Charter is part of public law, damages cannot be awarded in...
-
Data in the table come from a student experiment to measure the binding constant of the radioactively labeled hormone estradiol (X) to the protein, bovine serum albumin (P). Estradiol (7.5 nM) was...
-
What is the most common cause of conflict in today's workplaces?
-
Consider the following sample data: y 5 1 3 x 5 1 3 a. Construct a scatterplot for the data. LO9 b. It is possible to find many lines for which 1y - yn2 = 0. For this reason, the criterion 1y - yn2 =...
-
Textile Company frequently factors its accounts receivable. During 2007, Faeber made credit sales of $100,000 to customers, under terms of 2/10, n/30. Faeber records its credit sales using the gross...
-
Old Country Links, Inc., produces sausages in three production departmentsMixing, Casing and Curing, and Packaging. In the Mixing Department, meats are prepared and ground and then mixed with spices....
-
Develop cost functions for the PROJECT, UNION, INTERSECTION, SET DIFFERENCE, and CARTESIAN PRODUCT algorithms discussed in section19.4.
-
The language of accounting is littered with acronyms, abbreviations for common organizations or phrases. Match the phrase or organization on the left with its abbreviation. Phrase or Organizatlon...
-
Indicate whether the use of IFRS or ASPE is required or more likely for the following entities: IFRS ASPE 1. Bank 2. Private company two shareholders 3. Public company 4. Mutual fund 5. Private...
-
Calculate the iterated integral. C cos(x?) dy dx o Jo X. dx
-
Q10: Region ( experienced compressive stresses and has a than the rest of the bracket. Region ( ) experienced tension stresses and has a of the bracket. Deep Drawing and Stretch Forming width (into...
-
A sample of 1500 computer chips revealed that 32% of the chips do not fail in the first 1000 hours of their use. The company\'s promotional literature claimed that above 29% do not fail in the first...
-
The 75 lb block is released from rest 5 ft above the plate. Determine the compression of each spring when the block momentarily comes to rest after striking the plate. Neglect the mass of the plate....
-
Indiana Soy Products (OSP) buys soybeans and processes them into other soy products. Each ton of soybeans that OSP purchases for $250 can be converted for an additional $180 into 675 lbs of soy meal...
-
The 2025 Annual Report of Splish International contains the following informatio (in millions) June 29, 2025 June 27, 2024 Total assets $1,545 $1,502 Total liabilities 989 1,060 Net sales 2,800 2.971...
-
If v xb denotes the greatest integer in x, evaluate the integral where R = {(x, y) | 1 x 3, 2 y 5}. | [x + y] dA
-
Suppose Green Network Energy needs to raise money to finance its new manufacturing facility, but their CFO does not think the company is financially capable of making the periodic interest payments...
-
Give the relationships between the following pairs of structures. The possible relationships are: same compound, cis-trans isomers, constitutional (structural) isomers, not isomers (different...
-
Sulfur dioxide has a dipole moment of 1.60 D. Carbon dioxide has a dipole moment of zero, even though C-O bonds are more polar than S-O bonds. Explain this apparent contradiction.
-
For each of the following compounds, 1. Draw the Lewis structure. 2. Show how the bond dipole moments (and those of any nonbonding pairs of electrons) contribute to the molecular dipole moment. 3....
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App



