Many insecticides function by blocking cellular receptors for the insect molting hormone ecdysone. HO. HO CH3
Question:
Many insecticides function by blocking cellular receptors for the insect molting hormone ecdysone.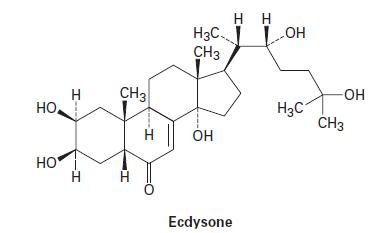
Transcribed Image Text:
HO. HO Н CH3 I H H3C CH3 OH Ecdysone Н Н -OH H3C -OH CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Many insecticides work by targeting and blocking the cellular receptors for ecdysone which is an ess...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The hormone estrogen is produced in the ovaries of females and elsewhere in the body in men and postmenopausal women, and it is also administered in estrogen replacement therapy, a common treatment...
-
Signaling by soluble extracellular molecules can be classified as endocrine, paracrine, or autocrine. Describe how these three types of cellular signaling differ. Growth hormone is secreted from the...
-
Suppose that the circulating concentration of hormone is 10 10 M and the Kd for binding to its receptor is 10 8 M. What fraction of the receptors will have hormone bound? If a meaningful...
-
Write the expression as one ratio without any negative exponents. x1/4x-3/4 X
-
Assume you are the manager of the finished goods ware-house of a stereo manufacturer. What costs are being incurred as stereos are stored while awaiting shipment to retail stores?
-
Use the laws of exponents to simplify the algebraic expressions. Your answer should not involve parentheses or negative exponents. 2x /x
-
Affording a Mercedes. A Mercedes-Benz 190 cost $24,000 in 1981, when the CPI (198284 = 100) was 90.9. The average CPI for 2007 was 207.3. How many 2007 dollars must you earn to have the same buying...
-
The adjusted seasonal indexes presented in Table P-19 reflect the changing volume of business of the Mt. Spokane Resort Hotel, which caters to family tourists in the summer and skiing enthusiasts...
-
A stock has a beta of 2.7, the market expected return is 8% and the riskfree rate is 2%. What is the expected rate of return according to CAPM? Express your answer as a percentage, for example 3.18%...
-
Give IUPAC names for the following acyl derivatives: (a) (d) CH3 CH3CHCH2CH2CCI (g) 0 0=0 H2C = CHCH2CH2CNHCH3 (b) (e) (h) CHCNH2 || OCHCH3 CH3 CH3 CH3CH2CHCN (c) (f) CH3CHCOCHCH3 CH3 CH3 CH3 CHCH3
-
Show the products that result from the reaction of phenylmagnesium bromide with the following reagents: (a) CH 2 O (b) Benzophenone (C 6 H 5 COC 6 H 5 ) (c) Pentan-3-one
-
On January 2, 2013, Whistler Company purchased land for $450,000, from which it is estimated that 400,000 tons of ore could be extracted. It estimates that the present value of the cost necessary to...
-
1. List at least three (3) ways a business can anticipate potential problems to prevent complaints. 2. Explain how to identify customer needs and expectations. 3. List at least five (5) ways to build...
-
you have taken over a company with 4 employees and you have 1 millon dollars with you.as business management student,you are expected to take 5 business decisions ensuring that the company is able to...
-
Draw the Diamond - E ( SERVO ) model of strategic management and provide ONE WORD ( or short phrase ) that best describes the relationships between the elements of the model. ( up to 1 0 points )
-
If a patient's X-ray is rejected (at the end of the 22-minute evaluation by the doctor), she has a second X-ray taken (assume that the second X-ray will always be accepted) and this new X-ray must be...
-
What are the cognitive appraisal processes involved in stress perception, and how can cognitive-behavioral techniques such as cognitive restructuring and mindfulness-based interventions help...
-
Use a graph to show how the analysis changes in the Challenge if Max and Bob view e-books and printed books as imperfect substitutes?
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
When butyl bromide is treated with sodium iodide in ethanol, the concentration of iodide quickly decreases but then slowly returns to its original concentration. Identify the major product of the...
-
In each of the following transformations, identify whether the starting material has been oxidized, reduced, or neither. Try to determine the answer without calculating Oxidation states, and then use...
-
Consider the structure of formaldehyde: a) Identify the type of bonds that form the C = O double bond. b) Identify the atomic orbitals that form each C = H bond. c) What type of atomic orbitals do...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...
-
1. Determine the value of the right to use asset and lease liability at commencement of the lease.
-
Problem 22-1 The management of Sunland Instrument Company had concluded, with the concurrence of its independent auditors, that results of operations would be more fairly presented if Sunland changed...

Study smarter with the SolutionInn App


