Methylation of tetrahydrofolate produces a cofactor called 5-methyltetrahydrofolate that is used in the conversion of homocysteine to
Question:
Methylation of tetrahydrofolate produces a cofactor called 5-methyltetrahydrofolate that is used in the conversion of homocysteine to methionine. Propose a mechanism for this reaction.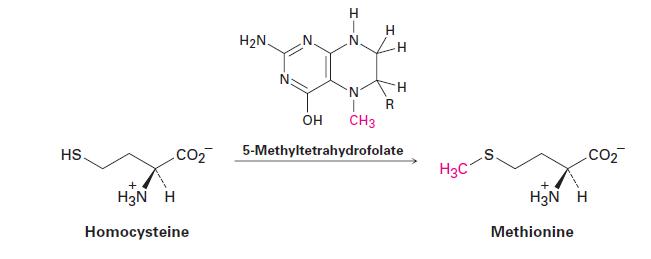
Transcribed Image Text:
HS. CO₂ H₂N H Homocysteine H₂N H T N I CH3 R H H OH 5-Methyltetrahydrofolate H3C S H₂N H Methionine CO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The conversion of homocysteine to methionine through the use of 5methyltetrahydrofolate involves a reaction known as methylation This reaction can be ...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What strategies can a company use to recover from ethical lapses?
-
A belt that is used in a drive mechanism in a copier machine is required to have a minimum tensile strength of LSL = 150 lb. It is known from long experience that = 5 lb for this particular belt....
-
You are working on your firms fifth audit of SSC. The previous audits have all resulted in standard unqualified audit reports. Read the following write-up from your audit files concerning SSC and its...
-
You are deciding between two mutually exclusive investment opportunities. Both require the same initial investment of $10 million. Investment A will generate $2 million per year (starting at the end...
-
Indicate whether each of the following variances is favorable or unfavorable. The first one has been done as anexample. Item to Classify Standard Actual Type of Variance Sales volume Sales price...
-
Specify the control word that must be applied to the processor of Fig. 8-2 to implement the following microoperations. a. R1R2 + R3 b. R4R4 c. R5R5 - 1 d. R6input Fig. 8-2 R1 R2 R3 R4 R5 R6 R7 Load...
-
The professor swims. Exercise 14.13 gives data on the time to swim 2000 yards and the pulse rate after swimming for a middle-aged professor. (a) Use a calculator to find the correlation r. Explain...
-
Consider a horizontal, thin-walled circular tube of diameter D = 0.025 m submerged in a container of n-octadecane (paraffin), which is used to store thermal energy. As hot water flows through the...
-
[The following information applies to the questions displayed below) Warnerwoods Company uses a perpetual inventory system. It entered into the following purchases and sales transactions for March...
-
In addition to transferring a methyl group, tetrahydrofolate can also transfer a formaldehyde group, a process that is critical for the biosynthesis of thymidine. Draw a mechanism for incorporation...
-
Many drugs function by interfering with metabolic pathways. The anticancer drug methotrexate, for instance, inhibits the dihydrofolate reductase enzyme that catalyzes the following reactions: What...
-
What risk management process is done during project execution? Provide an example.
-
Essay on: The Influence of Social Media on Individuals, Family, and Society. Also how social media influences us on a business end, how it influences us personally, and how they correlate.
-
A wastewater flow of 3550 m 3 /d is to be treated in a facultative pond system. The reaction rate coefficient at the average operating temperature is 0.35 d -1 . The pond system is assumed to behave...
-
Provide a brief overview using these environmental scanning steps as a guide: 1. Choose an industry of your interest such as technology, healthcare, retail, energy, etc. 2. Identify and briefly...
-
Your company is considering expanding into a new international market. Describe the market research you would conduct to evaluate the feasibility of entering this market, including factors such as...
-
Address the following from your Social Era research and the course scholarly literature: What are the key foundational underpinnings that shape the essence of what we call the social management era?...
-
The Homeland Security Act of 2002 is divided into 17 titles while bringing together under one umbrella more than 22 federal agencies. Select one such agency and describe their role and...
-
A liquid flows upward through a valve situated in a vertical pipe. Calculate the differential pressure (kPa) between points A and B. The mean velocity of the flow is 4.1 m/s. The specific gravity of...
-
Indicate whether you would use sodium ethoxide or potassium tert-butoxide to achieve each of the following transformations: a. b. c. d. Br Br
-
Explain why the following reaction yields the Hofmann product exclusively (no Zaitsev product at all) even though the base is not sterically hindered: Br NaOEt ELOH
-
Propose a mechanism for each of the following transformations: a. b.
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


