Nonconjugated , -unsaturated ketones such as cyclohex-3-enone are in an acid-catalyzed equilibrium with their conjugated , -unsaturated
Question:
Nonconjugated β, γ-unsaturated ketones such as cyclohex-3-enone are in an acid-catalyzed equilibrium with their conjugated α, β-unsaturated isomers. Propose a mechanism for the acid-catalyzed interconversion.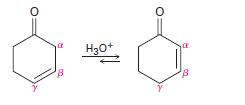
Transcribed Image Text:
H3O+ to
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The acidcatalyzed interconversion of nonconjugated unsaturated ketones to their conjugated unsaturat...View the full answer

Answered By

FELIX NYAMBWOGI
I have been tutoring for over 5 years, both in person and online. I have experience tutoring a wide range of subjects, including math, science, English, and history. I have also worked with students of all ages, from elementary school to high school.
In addition, I have received training in effective tutoring strategies and techniques, such as active listening, questioning, and feedback. I am also proficient in using online tutoring platforms, such as Zoom and Google Classroom, to effectively deliver virtual lessons.
Overall, my hands-on experience and proficiency as a tutor has allowed me to effectively support and guide students in achieving their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Non-conjugated ?, ?-un-saturated ketones, such as 3-cyclohexenonc, arc in an acid-catalyzed equilibrium with their conjugated a,-unsaturated isomers propose a mechanism for this isomerization. H30+
-
The , to , interconversion of unsaturated ketones is catalyzed by base as well as by acid. Propose a mechanism. Problem 11.29 Nonconjugated , -unsaturated ketones such as cyclohex-3-enone are in an...
-
Ketones and aldehydes react with primary amines to give imines. They react with secondary amines to give enamines (vinyl amines). (a) For review, propose a mechanism for the following formation of an...
-
In Exercises 7192, find and simplify the difference quotient f(x +h)-f(x) h -, h = 0
-
Over what period of time the cost of various types of intangible assets should be amortized by regular charges against revenue? (Your answer should be in the form of a principle or guideline rather...
-
The figure shows the graph of (x, y) = ln x + y. From the graph, does it appear that the limit at each point exists? (a) (-1, -1) (b) (0, 3) (c) (0, 0) (d) (2, 0) -8 - X -6 -4 86 Z -5-- 2 4 6 8 y
-
Calculate the conditional relative frequencies in the contingency table based on the row totals.
-
At Western University the historical mean of scholarship examination scores for fresh-man applications is 900. A historical population standard deviation = 180 is assumed known. Each year, the...
-
11. Komfy Karz is evaluating a project that costs $500,000 and is expected to generate $365,000 and $195,000, respectively, during the next two years. If Komfys required rate of return is 15%, what...
-
Write resonance structures for the following anions: (a) 0 0 |||| CH3CCHCCH3 (d) N=CCHCOCH5 (b) : CHC=N (c) = || CHCH=CHCHCCH3
-
Aldol condensation of butan-2-one leads to a mixture of two enones (ignoring double-bond stereochemistry). Draw them.
-
Beth was killed when a trench collapsed. An investigation revealed that the trench was 27 feet deep and without adequate shoring, in violation of safety standards. Bob, the president of the firm, is...
-
Write a java program that contain two overloaded methods that accepts two numbers or two characters representing a range example (11, 37) or (c, w) inputted by the user. The method generates a random...
-
Maggie could not conceive a child using natural means, so she sought out a woman who would donate an egg to be surgically implanted in Maggie. Which of the following items are deductible by Maggie in...
-
M corporation is subject to tax only in state b state b law provides for the use of federal taxable income before net operating loss and special deductions as the starting point for computing state...
-
Use Routh Criteria to determine the values of K needed for the system represented by the Characteristic Equation to be stable. (1 + K)s + (2K + 3)s + 2 3K = 0 Obtain the root locus plot for the...
-
Q7 a) Two forces equal to 2P and P act on a particle. If the first be doubled and second is increased by 12N, the direction of resultant remains unaltered. Find the value of P (5)
-
Remy spends her weekly income of $30 on chocolate, q1, and shampoo, q2. Initially, when the prices are p1 = $2 = p2, she buys q1 = 10 and q2 = 5. After the prices change to p1 = $1 and p2 = $3, she...
-
Problem 3.5 (4 points). We will prove, in steps, that rank (L) = rank(LT) for any LE Rnxm (a) Prove that rank (L) = rank (LTL). (Hint: use Problem 3.4.) (b) Use part (a) to deduce that that rank(L) =...
-
Predict the product of the following reaction. M 1) LIAID, 2) H20 Me
-
Epoxides can be formed by treating α-haloketones with sodium borohydride. Propose a mechanism for formation of the following epoxide. NABH,
-
When methyloxirane is treated with HBr, the bromide ion attacks the less substituted position. However, when phenyloxirane is treated with HBr, the bromide ion attacks the more substituted position....
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.

Study smarter with the SolutionInn App


