Predict the products of the reaction of the following substances with CrO 3 in aqueous acid: (a)
Question:
Predict the products of the reaction of the following substances with CrO3 in aqueous acid: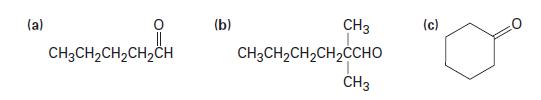
Transcribed Image Text:
(a) CH3CH₂CH₂CH₂CH (b) CH3 CH3CH,CH,CH, CHO CH3 (c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
To predict the products of the reactions with CrO3 in aqueous acid we need to consider the type of r...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What products would you expect from oxidation of the following compounds with CrO3 in aqueous acid with pyridinium chlorochromate? (a) 1-Hexanol (b) 2-Hexanol (c) Hexanol
-
Predict the products of the following acid-base reactions. Using pKa values, indicate which side of each equilibrium reaction is favored, and label the species representing the stronger acid and...
-
Name the following alkenes, and predict the products of their reaction with (1) meta-chloroperoxybenzoic acid, (ii) KMnO4 in aqueous acid, and (iii) O3, followed by Zn in aceticacid: (a) (b)
-
For each polynomial function, find (a) (-1), (b) (2), and (c) (0). f(x) = x + 2x - 8
-
Barnacle Industries was awarded a patent over 15 years ago for a unique industrial- strength cleaner that removes barnacles and other particles from the hulls of ships. Thanks to its monopoly...
-
In an electric circuit containing an inductance L and a capacitance C, the resonant frequency f is inversely proportional to the square root of the capacitance. If the resonant frequency in a circuit...
-
Service costing and process costing are the same.
-
Net present value , Internal Rate of Return, Sensitivity Analysis. Francesca Freed wants a Burg-N- Fry franchise. The buy-in is $500,000. Burg-N-Fry headquarters tells Francesca that typical annual...
-
Jackson Street Repairs stock currently sells for $55 per share. The market requires a 12% return on the firms stock. If the company maintains a constant 5% growth rate in dividends, what was the most...
-
How could you prepare hexan-2-one from the following starting materials? (a) (c) OH CH3CHCHCHCHCH3 CH3 CH3CHCHCHC=CH (b) CH3CHCHCHC=CH
-
Propose structures for molecules that meet the following descriptions: (a) A ketone, C 5 H 10O (b) An aldehyde, C 6 H 10O (c) A keto aldehyde, C 6 H 10 O 2 (d) A cyclic ketone, C 5 H 8 O (a) (d)...
-
The data are the same as in the previous exercise. Perform association rule analysis using the following settings. a. Generate association rules with a minimum support of 10 transactions and minimum...
-
Service provides commercial and industrial appraisals and feasibility studies. On January 1 , the assets and liabilities of the business were the following: Cash, \(\$ 8,700\); Accounts Receivable,...
-
Sketch the mapping of the value chain for: a A consulting firm b An airline c A trading firm d A corporate and investment bank e An internet-based platform (e.g. Airbnb, Netflix)?
-
Red River Banking Company has ten automatic i) AND teller machines (ATMs) spread throughout the city maintained by the ATM Department. You have been assigned the task of determining the cost of...
-
Super Day Spa provided \(\$ 120,000\) of services during 2012. All customers paid for the services with credit cards. Super submitted the credit card receipts to the credit card company immediately....
-
The following data represent the height of 26 statistics students as measured in inches: a. Create a frequency table for these data. b. Create a histogram for these data with an interval width of 1...
-
Can an indifference curve be downward sloping in one section, but then bend backward so that it forms a "hook" at the end of the indifference curve?
-
What is your opinion of advertising awards, such as the Cannes Lions, that are based solely on creativity? If you were a marketer looking for an agency, would you take these creative awards into...
-
Predict the multiplicity of each signal in the 1 H NMR spectrum of the following compound:
-
For each pair of compounds, identify how you would distinguish them using either 1 H NMR spectroscopy or 13 C NMR spectroscopy: (a) (b) (c) (d) 'CI CI CI
-
A compound with molecular formula C 8 H 18 exhibits a 1 H NMR spectrum with only one signal. How many signals would you expect in the 13 C NMR spectrum of this compound?
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


