Show the enolate ions you would obtain by deprotonation of the following carbonyl compounds: (a) 0 CH3CHCHCH
Question:
Show the enolate ions you would obtain by deprotonation of the following carbonyl compounds: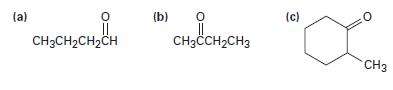
Transcribed Image Text:
(a) 0 CH3CH₂CH₂CH (b) 0 CH3CCH₂CH3 (c) 0 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
In the image provided the carbonyl compounds are Pentan2one 3Methylpentan2one 2Methylpentan3one Hexa...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What segment of the external environment has more impact on an organization? 2. If an organization wants to engage in a new industry, which area of the industry environment should be the most...
-
Show what alcohols and carbonyl compounds give the following derivatives. (a) (b) (c) (d) (e) (f) CH CH,O OCH,CH CH O-CH CH3 CH3 CH-C H O-CH CH a,0 OX
-
Show the resonance forms for the enolate ions that result when the following compounds are treated with a strong base. (a) Ethyl acetoacetate (b) Pentane-2,4-dione (c) Ethyl a-cyanoacetate (d)...
-
In Problems 1130, solve each equation by factoring. x 2 + 4x = 0
-
Translate the following amounts of foreign currency into an equivalent number of U.S. dollars using the exchange rates in Exhibit 15.7. a. 800,000 b. 350,000 c.50,000 Exchange Rate (in foreign...
-
Examine the function for relative extrema and saddle points. (x, y) = - x - 4y + 8x - 8y - 11
-
At a 0.01, perform a chi-square indepen- dence test to determine whether the variables response and gender are independent. What can you conclude? In Exercises 4 and 5, perform a chi-square...
-
(Single-step Income, Retained Earnings, Periodic Inventory) Presented below is the trial balance of Mary J. Blige Corporation at December 31, 2004. A physical count of inventory on December 31...
-
CSU, Inc., is a calendar year S corporation. CSUs Form 1120S shows nonseparately stated ordinary income of $120,000 for the year. Taewon owns 30% of the CSU stock throughout the year. The following...
-
Show how you might prepare pent-1-en-3-one from pentan-3-one: CH3CHCCHCH3 Pentan-3-one CH3CHCCH=CH Pent-1-en-3-one
-
How would you prepare the following ketones by reaction of a Grignard reagent and a nitrile? (a) || CH3CH2CCH2CH3 (b) ON CH3
-
Predict generally what youd expect to see in the SIR model results with respect to S, I, and R individuals. (Consider how these results would differ from not wearing masks.)
-
Based on the following information, calculate the sustainable growth rate for Kaleb's Welding Supply: Profit margin Capital intensity ratio Debt-equity ratio Net income Dividends 7.5% 0.65 0.60...
-
Waterway Inc. uses LIFO inventory costing. At January 1, 2025, inventory was $216,014 at both cost and market value. At December 31, 2025, the inventory was $283,252 at cost and $262,660 at market...
-
What is the 32-bit version of: 0000 0000 0001 0101
-
1. Let A = 2 1 4 3 Find AT, A-1, (A-1) and (AT)-1. 2. Let A = = [ -1 -1 2 22 (a) Find (AB), BT AT and AT BT. (b) (AB)-1, B-1A-1 and A-B-1. ] 1-5 and B = 1
-
Xavier Ltd. paid out cash dividends at the end of each year as follows: Year Dividend Paid 2018 $250,000 2019 $325,000 2020 $400,000 Assume that Xavier had 100,000 common shares and 5,000, $4,...
-
Draw a figure to illustrate the answer given in Solved Problem 4.3. Use math and a figure to show whether applying an ad valorem tax rather than a specific tax changes the analysis?
-
The Ranch 888 Noodle Company sells two types of dried noodles:ramen, at $6.50 per box, and chow fun, at $7.70 per box. So farthis year, the company has sold a total of 110,096 boxes ofnoodles,...
-
In each case below, identify the acid and the base. Then draw the curved arrows showing a proton transfer reaction. Draw the products of that proton transfer, and then predict the position of...
-
Identify the reagents you would use to accomplish each of the following transformations: Br Br -Br En Br En
-
In the previous problem, we saw that an acetylide ion can attack a variety of electrophiles. In Chapter 20, we will see that a C=O bond can also function as an electrophile. Consider the following...
-
Problem Set Time Value of Money 1. In 10 years, what is the value of $100 invested today at an interest rate of 8% per year, compounded annually? 2. In 10 years, what is the value of $100 invested...
-
The Blending Department of Luongo Company has the following cost and production data for the month of April. Costs: Work in process, April 1 Direct materials: 100% complete $120,000 Conversion costs:...
-
Q3 plz answer correctly and check work Builtrite's upper management has been comparing their books to industry standards and came up with the following question: Why is our operating profit margin...

Study smarter with the SolutionInn App


