Show the products obtained from addition of CH 3 MgBr to the following carbonyl compounds: (a) (b)
Question:
Show the products obtained from addition of CH3MgBr to the following carbonyl compounds: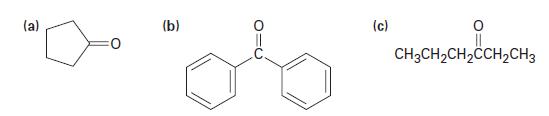
Transcribed Image Text:
(a) (b) (c) || CH3CH₂CH₂CCH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To determine the products obtained from the addition of CH3MgBr methylmagnesium bromide to the given ...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show the configuration of the products obtained from the following reaction: a. Under conditions that favor an SN2 reaction b. Under conditions that favor an SN1 reaction CH3OH
-
Show the products obtained from addition of methyl magnesium bromide to the following compounds: (a) Cyclopentanone (b) Benzophenone (diphenyl ketone) (c) 3-Hexanone
-
Show what alcohols and carbonyl compounds give the following derivatives. (a) (b) (c) (d) (e) (f) CH CH,O OCH,CH CH O-CH CH3 CH3 CH-C H O-CH CH a,0 OX
-
In Problems 5994, solve each inequality. Express your answer using set notation or interval notation. Graph the solution set. 2x2 3 + x
-
Andrew has decided to open an online store that sells home and garden products. After searching around, he chooses the software company Initech to pro-vide the software for his website, since their...
-
What types of training would Lynda.corn have difficulty providing customers? What kinds of education are less appropriate for e-leaming than traditional in-person courses? Lynda Weinman, cofounder of...
-
Living on campus. A February 2, 2008, article in the Columbus Dispatch reported a study on the distances students lived from campus and average GPA. Here is a summary of the results: Residence Avg....
-
The income statement accounts for the Monroe Realty Company at the end of its fiscal year are shown below. Prepare the required closing entries in journal form. Chris Ross is theowner. Account Name...
-
List the 5 Cs of credit and explain what a lending institution looks at to determine each C for a prospective borrower buying a home.
-
Predict the products you would expect from the following reactions. Indicate the major product in each case. (a) H3C OH TL CH3CHCCHCH3 T CH3 HSO4 (b) OH I CH3CHCHCCH3 CH3 HSO4
-
Alkane chlorination can occur at any position in the alkane chain. Draw and name all monochloro products you might obtain from radical chlorination of 3-methylpentane. Which, if any, are chiral?
-
Write out the first five terms (beginning with \(n=1\) ) of the sequences given in Problems 3-10. \(\left\{\frac{3 n+1}{n+2}ight\}\)
-
A large-sized chemical company is considering investing in a project that costs `5,00,000. The estimated salvage value is zero; tax rate is 35 per cent. The company uses straight line method of...
-
From the following budgeted and actual figures, calculate and present the variances in respect of profit on sales and cost of sales. Budget: Sales, 2,000 units @ 15 each Cost of sales @ 12 each...
-
(a) From the following data of a manufacturing unit, find out (i) sales to break-even and (ii) sales to earn a profit of 8,000. (b) The following information is available for companies A and B. (i)...
-
Wowem Corporation manufactures a wide range of clothing apparel. It is a decentralized organization in which different divisions have responsibility for the manufacture and distribution of major...
-
(a) Use a molecular orbital program or input and output from software supplied by your instructor to construct a molecular orbital energy-level diagram to correlate the MO (from the output) and AO...
-
A toroidal solenoid has an inner radius of 12.0 cm and an outer radius of 15.0cm. It carries a current of 1.50A. How many equally spaced turns must it have so that it will produce a magnetic field of...
-
As water moves through the hydrologic cycle, water quality changes are common because of natural phenomena or anthropogenic pollution. Using Figure 11.1, describe how water-quality changes occur...
-
Compound A and compound B are constitutional isomers with molecular formula C 3 H 7 Cl. When compound A is treated with sodium methoxide, a substitution reaction predominates. When compound B is...
-
The parameter a in the van der Waals equation is greater for H 2 O than for He. What does this say about the difference in the form of the potential function in Figure 1.10 for the two gases? Figure...
-
Compound A and compound B are constitutional isomers with molecular formula C 4 H 9 Cl. Treatment of compound A with sodium methoxide gives trans-2-butene as the major product, while treatment of...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


