Predict the products you would expect from the following reactions. Indicate the major product in each case.
Question:
Predict the products you would expect from the following reactions. Indicate the major product in each case.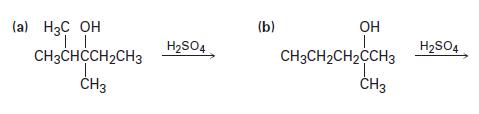
Transcribed Image Text:
(a) H3C OH TL CH3CHCCH₂CH3 T CH3 H₂SO4 (b) OH I CH3CH₂CH₂CCH3 CH3 H₂SO4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Lets predict the major products for each reaction Reaction 1 HBr red Br In this reaction HBr adds ac...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the products you would expect from the reaction of NaBH4 with the following compounds. You may assume that these reactions take place in methanol as the solvent. (a) CH3--(CH2)8--CHO (c) Ph...
-
Predict the products you expect when the following starting material undergoes oxidation with an excess of each of the reagents shown below. (a) Chromic acid (b) PCC (Pyridinium Chlorochromate) (c)...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
In Problems 67 90, multiply the polynomials using the special product formulas. Express your answer as a single polynomial in standard form. (x-4)
-
Recently, the owner of a Trader Joes franchise decided to change how she compensated her top manager. Last year, she paid him a fixed salary of $ 65,000, and her store made $ 120,000 in profits (not...
-
What are the benefits of telepresence robots for a company? When a robot resembling a vacuum cleaner topped with a computer monitor rolls by you a t work, you might at first think it is cleaning...
-
Measuring snakes. For a biology project, you measure the length (inches) and weight (grams) of 12 snakes. (a) Explain why you expect the correlation between length and weight to be positive. (b) If...
-
Selected accounts from the ledgers of Lockhart Company at July 31 showed the following. InstructionsFrom the data prepare:(a) The single-column purchases journal for July.(b) The general journal...
-
If the Fed injects a huge amount of money into the markets, inflation is expected to decline, and long - term interest rates are expected to rise. True or False?
-
How might you prepare the following substances by using nucleophilic substitution reactions? (a) CH 3 CH 2 CH 2 CH 2 OH (b) (CH 3 ) 2 CHCH 2 CH 2 N 3
-
Show the products obtained from addition of CH 3 MgBr to the following carbonyl compounds: (a) (b) (c) || CH3CHCHCCHCH3
-
J. D. Power, founder of J.D. Power and Associates, says, We define quality as what the customer wants. Do you agree or disagree with his observations? Explain your answer.
-
The equation for the standard normal curve (the normal curve with mean 0 and standard deviation 1) graphs as an exponential curve. Graph this curve, whose equation is \[y=\frac{e^{-x^{2} /...
-
Design an undirected network with N=7 and L=12. Based on how you drew your network, classify it as either fully connected ,random, or scale-free. Justify your decision with a short paragraph response.
-
Use the Ch08_AviaCo database shown in Figure P8.35 to work Problems 3546. Modify the MODEL table to add the attribute and insert the values shown in the following table. Table P8.35 Attribute and...
-
The Tip Calculator app does not need a Button to perform its calculations. Reimplement this app to use property listeners to perform the calculations whenever the user modifies the bill amount or...
-
A particle, carrying a positive charge of \(4 \mathrm{nC}\), located at \((5 \mathrm{~cm}, 0)\) on the \(x\)-axis experiences an attractive force of magnitude 115.2 \(\mathrm{N}\) due to an unknown...
-
A volume of 18.72 mL of 0.1500M K2Cr2O7 solution was required to titrate a sample of FeSO4 according to the equation in Problem 7.96. What is the mass of the sample?
-
(a) What do data breach notification laws require? (b) Why has this caused companies to think more about security?
-
When 2-chloro-1, 1, 2, 3, 3-pentamethylcyclohexane is treated with sodium hydroxide, neither E2 nor S N 2 products are formed. Explain.
-
Why are the triple point temperature and the normal freezing point very close in temperature for most substances?
-
Identify the major and minor product(s) that are expected for each of the following reactions: a. b. c. d. e. f. g. h. i. j. k. l. m. n. OTs Naci DMSO NaOH
-
Suppose First Fidelity Bank engaged in the following transactions: (Click the icon to view the transactions.) Journalize the 2018 and 2019 transactions on First Fidelity's books. Explanations are not...
-
Financial data for Joel de Paris, Inc., for last year follow: Joel de Paris, Inc. Balance Sheet Beginning Balance Ending Balance Assets Cash Accounts receivable Inventory Plant and equipment, net...
-
Supply costs at Coulthard Corporation's chain of gyms are listed below: March April May June July August September October November Client-Visits 11,666 11,462 11,994 13,900 11,726 11, 212 12,006...

Study smarter with the SolutionInn App


