Tell whether each of the following compounds has the E or the Z configuration: a. b. c.
Question:
Tell whether each of the following compounds has the E or the Z configuration:
a.
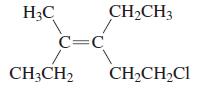
b.
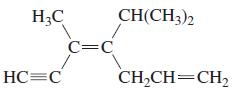
c.
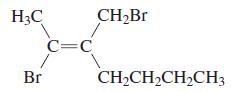
d.
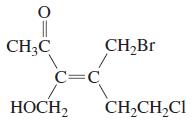
Transcribed Image Text:
H3C CH2CH3 C=C CH3CH2 CH2CH2CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
The E Z configurations can be identified by observation of the compounds attache...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine whether each of the following compounds is a cis isomer or a trans isomer a. b. c. d. e. f. Cl Br CH3 CH3 Br Br CH3 Cl CH3 CH3 CH
-
Tell whether each of the following molecules has a meso stereoisomer. (a) (b) CH CHCH CHCH Cl CH, CHCH2CH,CH CI
-
Identify whether each of the following compounds is chiral or achiral: a. b. c. d. e. f. g. h. i. j. k. l. m. n. o. p. CI
-
Charles has a savings account with a balance, today, of 100,000 SAR in his investment account. He expects to obtain a yearly return of 12% in his investments. How long will it take for Charles to...
-
(a) The mean free path is the average distance a molecule travels before colliding with another molecule. The mean free path,, is given = KTI (2P) by where k is Boltzmann's constant, T is...
-
What pressures to change can you identify in each company case? What pressures, if any, are similar across all four companies? What, if any, are different? What can we learn from this? AppendixLO1
-
Calculate SSE and s2 for each of the following cases: LO9 a. n = 25, SSyy = 110, SSxy = 60, b n 1 = .80 b. n = 50, ay2 = 900, ay = 90, SSxy = 2500, b n 1 = .25 c. n = 25, a1yi - y22 = 75, SSxy = 100,...
-
Use the methods of descriptive statistics to learn about the customers who visit the Heavenly Chocolates website. Include the following in your report. 1. Graphical and numerical summaries for the...
-
Acme does not issue its quarterly financial statements until nine months after the end of the quarter.
-
An LBO group is making an offer to purchase a target company. The company's current stock price is $9.50 per share, and the company has 250 million shares outstanding; the company currently has...
-
The rate constant for a reaction can be increased by _____ the stability of the reactant or by _____ the stability of the transition state.
-
Which of the following carbocations would you expect to rearrange? a. b. CH2 CH3 CH3 e. CH;CHCHCH3 .
-
Describe some specific acts that a trial judge could justifiably consider to be direct contempts of court.
-
Identify a public conflict (such as a recent Congressional debate or even a celebrity breakup) that has come to the forefront in the media (or public's attention) in the last thirty days. You have...
-
Performance Management Issues You have been asked to return to your alma mater and speak to current students about performance management issues. To make the most of this experience for yourself and...
-
Analysis of competitor organization of our selected organization Walmart and its competitor Safeway. 1. Complete analysis of competitor organization; addresses all relevant factors and typically uses...
-
Defining Program Objectives of Youth centers Clearly define the objectives of your program or center. What specific outcomes do you hope to achieve? Examples may include promoting physical fitness,...
-
Identify a local or regional organization and analyze how they demonstrate servant leadership in their operations. You will want to review their website, social media, news, and other resources to...
-
Use geometry or symmetry, or both, to evaluate the double integral. D = [a, a] [b, b] (ax + by + Ja x ) dA, D
-
1. As a general strategy, would you recommend that Carl take an aggressive approach to capacity expansion or more of a wait-and-see approach? 2. Should Carl go with the option for one facility that...
-
Tributylamine has a Cl mass spectrum with a strong M + 1 peak and one other major peak resulting from a-cleavage. At what m/z value does this peak occur?
-
In the warehouse of the company Tumany Amines, Iilc., two unidentified compounds have been found. The president of the company, Wotta Stench, has hired you to identify them from their spectra:...
-
In the warehouse of the company Tumany Amines, Iilc., two unidentified compounds have been found. The president of the company, Wotta Stench, has hired you to identify them from their spectra:...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


