Tert-Butyl ethers react with trifl uoroacetic acid, CF 3 CO 2 H, to yield an alcohol and
Question:
Tert-Butyl ethers react with trifl uoroacetic acid, CF3CO2H, to yield an alcohol and 2-methylpropene. Tell what kind of reaction is occurring, and propose a mechanism.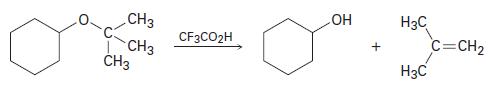
Transcribed Image Text:
CH3 CH3 CH3 CF3CO2H + H3C H3C C=CH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The reaction of tertbutyl ethers with trifluoroacetic acid CF3CO2H to yield an alcohol and 2methylpr...View the full answer

Answered By

Deepak Sharma
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1, 3-Cyclopentadlene reacts with Cycloheptatrienone to give the product shown. Tell what kind of reaction is Involved, and explain the observed result. Is the reaction suprafacial orantarafacial?...
-
On standing, 1,3-cyclopentadiene is transformed into a new compound called dicyclopentadiene, having the molecular formula C10H12. Hydrogenation of dicyclopentadiene gives the compound shown. Suggest...
-
A study by the Pew Internet and American Life Project (pewinternet.org) found that Americans had a complex and ambivalent attitude toward technology. (Data extracted from M. Himowitz, "How to Tell...
-
What is project scoping? Why is it important to good problem solving?
-
The Hull Petroleum Company and Inverted V are retail gasoline franchises that compete in a local market to sell gasoline to consumers. Hull and Inverted V are located across the street from each...
-
Solve for x: |x 5| < 3 and |x 7| < 2.
-
Shriman operates a taxi. Compute cost per running km from the following details. Rs Purchase price of taxi 50,000 Insurance per annum 1,000 Rent of garage per month 100 Tyres and tubes per set (A set...
-
The Sherill Utility District was recently established. Its balance sheet, after one year, is presented below. Note the following additional information: ¢ The general fund received all of its...
-
Choose a Canadian company and explain impact of trade agreements on competitive advantage of the company.
-
How would you prepare the following compounds from 2-phenylethanol? (a) Benzoic acid (b) Ethylbenzene (c) 1-Bromo-2-phenylethane (d) Phenylacetic acid (C 6 H 5 CH 2 CO 2 H) (e) Phenylacetaldehyde (C...
-
But-2-ene-1-thiol is a component of skunk spray. How would you synthesize this substance from but-2-en-1-ol? From methyl but-2-enoate, CH 3 CH=CHCO 2 CH 3 ? More than one step is required in both...
-
Using the following Latimer diagram, which shows the standard potentials for sulfur species in acid solution (pH = 0), construct a Frost diagram and calculate the standard potential for the HSO 4 /S...
-
The amounts of caffeine in a sample of five-ounce servings of brewed coffee are shown in the histogram. Number of 5-ounce servings S 25- 20 15 10 25 12 10 1 2 70.5 92.5 114.5 136.5 158.5 Caffeine (in...
-
Tom, David, Dale, and Murdock are four business students who want to rent a four- bedroom apartment together for the fall semester. They have identified the three factors important to them in...
-
Listed below, out of order, are the steps in an accounting cycle. 1. Prepare the unadjusted trial balance. 2. Post journal entries to general ledger accounts. 3. Analyze transactions from source...
-
Consider Quick Start QFD Matrix 2 above. Which two technical specifications are strongly correlated with each other? Quick Start QFD Matrix 2 Strong positive correlation Some positive correlation ==...
-
A cylindrical solenoid of length \(\ell\) and radius \(R\) has \(n\) windings per unit length and carries a current \(I\). (a) Use the inductance expression \(L=\left(\mu_{0} N^{2} A ight) / \ell\)...
-
If the demand function is Q = 110 - 20p, and the supply function is Q = 20 + 10p, what are the equilibrium price and quantity?
-
Suppose that the electrical potential at the point (x, y, z) is E(x, y, z) = x + y - 2z. What is the direction of the acceleration at the point (1,3,2)?
-
Draw all possible conjugated dienes with molecular formula C 6 H 10 , taking special care not to draw the same compound twice.
-
Treatment of 1,2-dibromocycloheptane with excess potassium tert-butoxide yields a product that absorbs UV light. Identify the product.
-
In each of the following pairs of compounds identify the compound that liberates the most heat upon hydrogenation. (a) (b)
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


