What carbonyl compounds would you reduce to prepare the following alcohols? List all possibilities. (a) CH3 CH3CHCHCHCCHOH
Question:
What carbonyl compounds would you reduce to prepare the following alcohols? List all possibilities.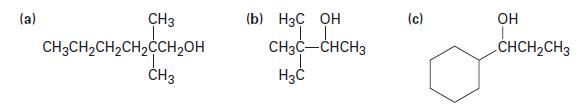
Transcribed Image Text:
(a) CH3 CH3CH₂CH₂CH₂CCH₂OH CH3 (b) H3C OH CH3C-CHCH3 H3C (c) OH CHCH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To prepare the alcohol CH3CH2CH2CH2CCH2OH you would need to reduce a carbonyl compo...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What carbonyl compounds would you reduce to prepare the following alcohols? List all possibilities. (b) CH |(a) (c) H CHCH2CH2H2H2 C CHCH-CH
-
What carbonyl compounds might you start with to prepare the following compounds by Grignard reaction? List all possibilities. (a) 2-Methyl-2-propanol (b) 1-Ethylcyclohexanol (c) 3-Phenyl-3-pentanol...
-
Show what alcohols and carbonyl compounds give the following derivatives. (a) (b) (c) (d) (e) (f) CH CH,O OCH,CH CH O-CH CH3 CH3 CH-C H O-CH CH a,0 OX
-
Problems 113 122. The purpose of these problems is to keep the material fresh in your mind so that you are better prepared for later sections, a final exam, or subsequent courses such as calculus. X...
-
The French government announced plans to convert state- owned power firms EDF and GDF into separate limited companies that operate in geographically distinct markets. BBC News reported that Frances...
-
Draw a sketch of the graph of the region in which the points satisfy the given system of inequalities. |y + 2| < 5 |x 3| 2
-
Sohan Singh has started transport business with a fleet of 10 taxis. The various expenses incurred by him are given below: (a) Cost of each taxiRs 75,000 (b) Salary of office staffRs 1,500 p.m. (c)...
-
Each year, ratings are compiled concerning the performance of new cars during the first 90 days of use. Suppose that the cars have been categorized according to whether a car needs warranty-related...
-
ABC Manufacturing Inc. ends the month with two jobs still in progress. Job 5 has $10,000 of materials, $2,000 of direct labor and $8,000 of manufacturing overhead allocated. Job 6 has $30,000 of...
-
Which ion in each of the following pairs is a better leaving group? (a) F - or Br - (b) Cl - or NH 2 - (c) OH - or I -
-
Describe the effects of the variables on both S N 2 and S N 1 reactions: (a) Substrate structure (b) Leaving group
-
The lottery ticket variable discussed in this chapter (Hardoon et al., 2001) examined the ticket preferences of nonproblem gamblers. One of the purposes of this study was to compare the preferences...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a converging lens, closer to the lens than to the focal point. (b) Is the image...
-
Power efficiency has become very important for modern processors, particularly for embedded systems. Create a version of gcc for two architectures that you have access to, such as x86, RISC-V,...
-
There is a movement toward wireless mobile computing using thin-client technology. Go to the Web and visit some of the ma jor computer vendors that are producing thin-client products such as handheld...
-
Draw a B-tree of order 4 and height 3 containing the fewest elements. Show an example of a split that would be applied by inserting the fewest number of elements.
-
Repeat Example 10-4, except calculate the diameter at the bottom of the column. Example 10-4 A distillation column is separating n-hexane from n-heptane using 1-in. ceramic Intalox saddles. The...
-
Using the estimated demand function for processed pork in Canada, Equation 2.2, show how the quantity demanded, Q, at a given price changes as per capita income, Y, increases slightly (that is,...
-
The nitrogen atoms in N2 participate in multiple bonding, whereas those in hydrazine, N2H4, do not. (a) Draw Lewis structures for both molecules. (b) What is the hybridization of the nitrogen atoms...
-
The cyclopropenyl cation has a three-membered ring that contains a continuous system of overlapping p orbitals. This system contains a total of two Ï electrons. Using a Frost circle, draw an...
-
Arrange each set of isomeric alkenes in order of stability. a. b.
-
Consider the following two isomeric alkenes. The first isomer is a mono-substituted alkene, while the second isomer is a di-substituted alkene. We might expect the second isomer to be more stable,...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


