What carbonyl compounds would you reduce to prepare the following alcohols? List all possibilities. (b)
Question:
What carbonyl compounds would you reduce to prepare the following alcohols? List all possibilities.
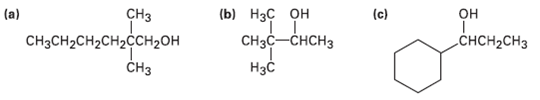
Transcribed Image Text:
(b) Нзс он Cнзс—снсHз Нас |(a) (c) СHз CHзCH2CH2сH2ссH2он CНз он CHCH-CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 46% (13 reviews)
a b c Alcohol CH3 CH3CHCHCHCCHOH ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What alkenes might be used to prepare the following alcohols by hydroboration/oxidation? (a) CH3 (b) CHH (c) .CH- CHCHCH2CH2OH
-
What reagents would you use to prepare the following compounds? a. b. CH CCH CH:C 0 CH3CCH-CH-CH (COCH-CH3 )2
-
What alkyl halides would you use to prepare the following ketones by an acetoacetic ester synthesis? CH (a) (b) CHH,H,H3 -CH-CH2CH2CCH
-
Chambers Corporation purchased a piece of equipment for $36,000. It estimated a 6-year life and $6,000 salvage value. Thus, straight-line depreciation was $5,000 per year [($36,000 $6,000) 6]. At...
-
Describe SWOT analysis as a way to guide internal analysis. How does this approach reflect the basic strategic management process?
-
Draw the influence line for the moment at A. Plot numerical values at the peaks. Assume A is fixed and the support at B is a roller. EI is constant. 3 m 3 m
-
9. How is the amount of piecemeal recognition of constructive gain or loss determined in the intercompany bonds transaction?
-
1. How does technology sustain Megan Ducketts business? 2. Why does Megan credit computer technology and the Web for a significant portion of her companys growth? 3. What have been some critical...
-
Assume you are working with the accounting department in your organization to make a decision regarding a capital investment you feel is needed to improve productivity in your department. The...
-
A Global private bank is aggressively looking to leverage technology to improve customer experience and reduce operational costs. Over the last few years, it has tied up with at least five startups...
-
What Grignard reagent and what carbonyl compound might you start with to prepare the following alcohols? CH (a) (b) (c) CHCH-CHCH2CH CHH2H H2c=C "CH- (d) (e) (f) .CH- "
-
How would you carry out the following transformations? Co .CO2H (a) (b) Co CH2 C (c) CH2SH
-
Orion, Inc., a U.S. corporation, reports foreign-source income and pays foreign taxes for the tax year as follows. Orion's worldwide taxable income is $600,000, and U.S. taxes before the ITC are...
-
Assume a Poisson distribution with =5.6. Find the following probabilities. a. X=1 b. X <1 c. X>1 d. X1 a. P(X=1)= (Round to four decimal places asneeded.) b. P(X <1)= (Round to four decimal places...
-
345879 The any reported the following January purchases and sales data for its only prauct. The company uses a perpetual inventory system. REQUIRED: Determine the cost assigned to ending inventory...
-
How do changing geopolitical landscapes, such as shifting alliances and emerging power centers, influence conflict resolution strategies, and what adjustments are necessary to address new global...
-
50 21 2. Determine the inclination and period of the satellite which produced the ground trace below. Show all calculations. Suteite 17 11-140-130-120-110 tonn an 20 6058 am 50 210 0 10 20 30 50 60...
-
This activity aims to provide practical experience in preparing tax forms related to business income and depreciation. It emphasizes the importance of accurate reporting and adherence to tax...
-
How do these volunteers fit into an organizations structure? Take each of the six elements of organizational design (see Chapter 10, pages 321332) and discuss how each would affect this structural...
-
According during to the IRS, individuals filing federal income tax returns prior to March 31 received an average refund of $1,088 in 2018. Consider the population of "last-minute" filers who mail...
-
In a capillary rise experiment, the height (h) to which a liquid rises depends on the density (d) and surface tension () of the liquid and the radius of the capillary (r). The equation relating these...
-
Chloramphenicol (at right) is a potent antibiotic, isolated from Streptomyces venezuelae, that is particularly effective against typhoid fever. It was the first naturally occurring substance shown to...
-
(a) Give the (R,S) designations for each chirality center in compound A and for compound B. (b) Write the Fischer projection formula for a compound C that is the diastereomer of A and B. (c) Would C...
-
(a) Is trans-1, 2-dimethylcyclopentane (5) superposable on its mirror image (i.e., on compound 6)? (b) Is cis-1, 2-dimethylcyclopentane (7) superposable on its mirror image? (c) Is cis-1,...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


