(a) Although we normally think of acetic acid as an acid, it is amphoteric and can also...
Question:
(a) Although we normally think of acetic acid as an acid, it is amphoteric and can also act as a weak base. The conjugate acid of acetic acid is shown below. Using the curved-arrow notation, derive a resonance structure for this ion which, taken with the structure below, shows that the two—OH groups are equivalent, the two C—O bonds are equivalent, and the positive charge is shared equally by the two oxygens. Draw a single hybrid structure for this ion using dashed lines and partial charges that conveys the same idea.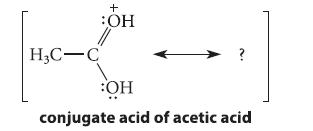
(b) The resonance structures of carbon monoxide are shown below. Show how each structure can be converted into the other using the curved-arrow notation.![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





