Which of the following are electron-deficient compounds? Explain. (a) (c) (e) HC CH3 C* CH3 1+ HC-N-CH3
Question:
Which of the following are electron-deficient compounds? Explain.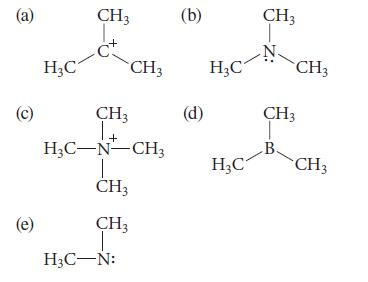
Transcribed Image Text:
(a) (c) (e) H₂C CH3 C* CH3 1+ H₂C-N-CH3 CH3 CH3 T CH 3 H₂C-N: (b) (d) H₂C H₂C CH3 CH3 CH3 B. CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a The carbon of this ion is electrondeficient because it has a sextet of ele...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An investor wishes to analyse the effects of different compounding frequencies Suppose 1000 is invested for 1 year at an interest rate of 5 per annum compounded Assume there are 365 days in 1 year
-
Which of the following are elements, which are molecules but not compounds, which are compounds but not molecules, and which are both compounds and molecules? (a) SO2, (b) S8, (c) Cs, (d) N2O5, (e)...
-
Which of the following compounds are aromatic? a. b. c. Cycloheptatrienyl cation d. e. f. g. Cyclononatetraenyl anion h. CH2=CHCH=CHCH=CH2
-
(a) Using your knowledge of economics and how markets work illustrate and explain why the price of electricity has increased so much over the past 18 months throughout the EU. You should consider the...
-
Did Dr. Moore have a duty to Tatiana Tarasoff, and did he breach that duty?
-
Carbon dioxide, which is recognized as the major contributor to global warming as a "greenhouse gas," is formed when fossil fuels are combusted, as in electrical power plants fueled by coal, oil, or...
-
Why would it be difficult to evaluate whether or not a project achieved its MOV shortly after the product is released or the system is implemented? AppendixLO1
-
The Chartered Financial Analyst (CFA) designation is fast becoming a requirement for serious investment professionals. It is an attractive alternative to getting an MBA for students wanting a career...
-
On July 1, 2020, Blue Corporation purchased Young Company by paying $251,500 cash and issuing a $130,000 note payable to Steve Young. At July 1, 2020, the balance sheet of Young Company was as...
-
(a) Although we normally think of acetic acid as an acid, it is amphoteric and can also act as a weak base. The conjugate acid of acetic acid is shown below. Using the curved-arrow notation, derive a...
-
In each of the following sets, arrange the compounds in order of decreasing pK a , and explain your reasoning. (a) CICHCHSH CICHCHOH CH3CHOH (b) (c) _CH_i_OH HC-C-OH CH3O-CH-C-OH + :OH || HC-C-OH A +...
-
A hydraulic jump occurs in a rectangular channel. The flow rate is \(50 \mathrm{~m}^{3} / \mathrm{s}\) and the depth before the jump is \(2 \mathrm{~m}\). Determine the depth after the jump and the...
-
PROVIDE A CASE BRIEF FOR THE FOLLOWING CASE PROVIDED BELOW: PEOPLE v. REKTE Court of Appeal, Fourth District, Division 2, California. The PEOPLE, Plaintiff and Respondent, v. Viktors Andris REKTE,...
-
The time between release from prison and another crime charge for a certain group of men is 36 months with a standard deviation of 9 months. What percentage of men get charged with a second crime...
-
capacitance simulation: https://phet.colorado.edu/sims/html/capacitor-lab-basics/latest/capacitor-lab-basics_en.html w Lab 4 (1).docx Homework Help - Q&A from Or x + C...
-
Mel Jackson, a resident of Tennessee, has been a driver for Blues Delivery Company for the past 7 years. For this purpose, he leases a truck from Blue, and his compensation is based on a percentage...
-
On Halloween night, a small boy decided to dress up as a bank robber. He went to house where the lights were on, indicating that the owner was receiving trick-or-treaters. When the homeowner, a...
-
List five factors that control when and if a cell divides.
-
If a test has high reliability. O the test measures what the authors of the test claim it measures O people who take the same test twice get approximately the same scores both times O scores on the...
-
Tell whether each of the following substituents on a steroid is axial or equatorial. (A substituent that is ?up? is on the top face of the molecule as drawn, and a substituent that is ?down? is on...
-
Amantadine is an antiviral agent that is active against influenza A infection and against some strains of H5N1 avian flu. Draw a three-dimensional representation of amantadine showing the chair...
-
Ketones react with alcohols to yield products called acetals. Why does the allcis isomer of 4-tert-butyl-l, 3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal while...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


