In each of the following sets, arrange the compounds in order of decreasing pK a , and
Question:
In each of the following sets, arrange the compounds in order of decreasing pKa, and explain your reasoning.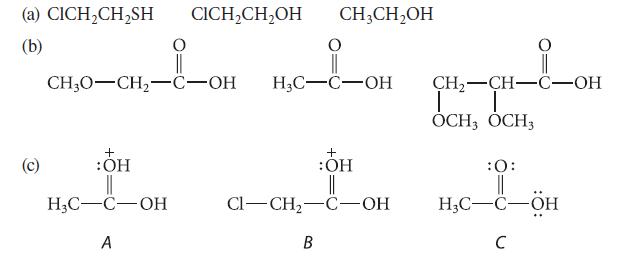
Transcribed Image Text:
(a) CICH₂CH₂SH CICH₂CH₂OH CH3CH₂OH (b) (c) _CH_i_OH HC-C-OH CH3O-CH₂-C-OH + :OH || H₂C-C-OH A + :OH || CI-CH₂-C-OH B CH₂-CH-C-OH T │ OCH, OCH3 :0: TI H3C-C-ÖH C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
In each set label the compounds from left to right as A B and C a The order ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the compounds in each of the following sets in order of decreasing pKa, highest first. Explain your reasoning. (a) CLCH2CH2SH CH3CH2OH CH3CH2SH (b) CH,CH,OH (CH3),N-CH-CH,OH (CH3)N OH
-
Arrange the compounds in each of the following groups in order of increasing solubility in water, and briefly explain your answers: a. 1-octanol; ethanol; ethyl chloride b. HOCH2(CHOH)3CH2OH;...
-
Arrange the bonds in each of the following sets in order of increasing polarity: (a) C-F, O-F, Be-F (b) O-Cl, S-Br, C-P (c) C-S, B-F, N-O
-
Use the data below to compute for the Gini coefficient. Round off the Gini coefficient to four (4) decimal places. INCOME CLASS (K) FIRST SECOND THIRD FOURTH FIFTH INCOME SHARE (YK) 0.02 0.12 0.28...
-
Does the First Amendment protect flag burning?
-
Nickel carbonyl, Ni (CO)4, is one of the most toxic substances known. The present maximum allowable concentration in laboratory air during an 8-hr workday is 1 ppb (parts per billion) by volume,...
-
Why should any lessons learned from project evaluations be documented? AppendixLO1
-
Northwest Aircraft Industries (NAI) was founded 45 years ago by Jay Preston as a small machine shop producing machined parts for the aircraft industry, which is prominent in the Seattle/Tacoma area...
-
3 A company is considering the purchase of a machine that w 140.000 do machine would have a salvage value of $43.000 The machine would reduce border DOO working capital of $5,000 would be needed...
-
Which of the following are electron-deficient compounds? Explain. (a) (c) (e) HC CH3 C* CH3 1+ HC-N-CH3 CH3 CH3 T CH 3 HC-N: (b) (d) HC HC CH3 CH3 CH3 B. CH3
-
Complete each of the following statements with a number. Assume that the temperature is 25C (298 K). (a) Two reactions have equilibrium constants that differ by a factor of 10. Their standard free...
-
Assume that you have to prepare older employees with little computer experience to attend a training course on how to use the Internet. How will you ensure that they have high levels of readiness for...
-
Activity 1.4: When Less Becomes More For this activity, refer to the images shown. This is an activity which was performed for you if you do not have available two identical mirrors at home. But if...
-
! Required information [The following information applies to the questions displayed below.] Aces Incorporated, a manufacturer of tennis rackets, began operations this year. The company produced...
-
During the early part of winter, one morning, two hunters decided to go quail hunting on a property where the owner had given them permission to hunt. A nearby forest ranger saw the hunters and...
-
Required information [The following information applies to the questions displayed below.] Trini Company set the following standard costs per unit for its single product. Direct materials (30 pounds...
-
A horticulturist knows that the weights of honeybees that have previously visited her orchard are normally distributed with a mean of 0.87 grams, and a population standard deviation of 0.15 grams....
-
Describe the events of mitosis in sequence.
-
a. What is meant by the term tax haven? b. What are the desired characteristics for a country if it expects to be used as a tax haven? c. What are the advantages leading an MNE to use a tax haven...
-
Alcohols undergo an oxidation reaction to yield carbonyl compounds on treatment with CrO 3 . For example, 2-tert-butylcyclohexanol gives 2-tert-butylcyclo- hexanone. If axial ?OH groups are generally...
-
Classify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) CH3Br + KOH CH3OH + KBr (b) CH3CH2Br H2C = CH2 + HBr (c) H2C = CH2 + H2 CH3CH3
-
Radical chlorination of alkanes is not generally useful because mixtures of products often result when more than one kind of CH bond is present in the substrate. Draw and name all monochloro...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


