(a) Complete the series of reactions in Fig. P19.50 by giving the major organic product. (b) Show...
Question:
(a) Complete the series of reactions in Fig. P19.50 by giving the major organic product.
(b) Show how the same product could be prepared from hydroquinone monomethyl ether (p-methoxyphenol).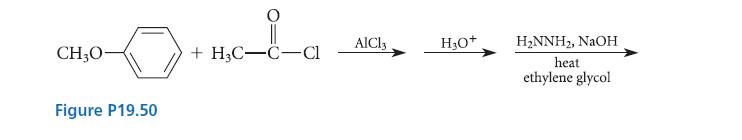
Transcribed Image Text:
CH₂0- Figure P19.50 +H3C-C-Cl AlCl3 H₂O+ H₂NNH₂, NaOH heat ethylene glycol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 16% (6 reviews)
a FriedelCrafts acylation gives a ketone pmethoxyacetophenone which reacts i...View the full answer

Answered By

Kennedy Odhiambo
As a professional writer, I have been in the field for over 5 years having worked as a lecture in different tertiary institutions across the world. With this impeccable experience, I assure provision of a good and supporting environment for students to learn.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show how the following compounds could be prepared from the given starting materials. You can use any necessary organic or inorganic reagents. a. b. c. d. e. f. CH3CH2CNH2 C CH.CH-CH-CH-OH-....
-
Show how the following compounds could be prepared from benzene: a. b. c. CH2 OCH3 Br NO2 CH3 SO H CH2CH CH3)2
-
Show how the following compounds could be prepared from cyclohexanone 0 CCH2CH2CH3 CH CCH3 CH2CH2CH3 CH CH2CH3 CH3
-
The profit before tax as reflected in the draft statement of comprehensive income of Sword Limited for the financial years ended 31 December 2020 and 31 December 2021 respectively was as follows:...
-
After closing its accounts at July 31, 2012, Goodrow Electric Company had the following account balances: Requirement 1. Prepare Goodrows post-closing trial balance at July 31,2012. Long-term...
-
Alberto-Culver Co., a Delaware corporation with its principal office in Illinois, manufactured toiletries and hair products in the United States and abroad. In February 1969, Alberto-Culver signed a...
-
More NCAA rules. For Division I athletes the NCAA uses a sliding scale, based on both core GPA and the combined Mathematics and Critical Reading SAT score, to determine eligibility to compete in the...
-
The Bango Toy Company produces several types of toys to seasonal demand. The forecast for the next six months in thousands of dollars is given below: A regular employee can produce $10,000 worth of...
-
A continuous random variable X has a pdf of the form: f(x)=(251/896)x2, for 0.88
-
Which of the following is not a good thing to do during an interview? a. Conduct the interview in private. b. Establish the purpose of the interview. c. Interview more than one person at once. d. Do...
-
Using known reactions and mechanisms discussed in the text, complete the reactions given in Fig. P19.49. (a) (b) i (CH3CHCH)C-C-CHCH3 PhP i CH3CHCHCCH3 + HN-Ph NaBH4 CH3OH NaH NaBH4 CH3OH
-
Write parametric equations for the following curves. Solutions are not unique. The right side of the ellipse x 2 /9 + y 2 /4 = 1, generated counterclockwise
-
Case study: Sun City - improving operations performance to enhance guest experience 1. Describe how Sun City implements the five operations performance objectives or principles. 2. Using your...
-
What recommendations do you have to increase the likelihood of success? E.g., how would you reduce the likelihood of having to go back to A4? How would you reduce the impact of having to go back to...
-
Problem 4 An electrically heated, square plate (0.4mx 0.4 mx0.005 m) is suspended in air of temperature Too = 20C. Find the electrical power needed to maintain the plate at T=95C if the plate is (a)...
-
Number of units Unit Cost Sales Beginning inventory 800 $50 Purchased 600 $52 Sold 400 $80 Sold 350 $90 Ending inventory 650 In the table below, calculate the dollar value for the period for each of...
-
10. Dr. D went to MGM Springfield casino while the class was taking their midterm exam. He played a Konami machine entitled 88 Fortunes. A slot attendant accidently left the slot manual next to the...
-
Write down a maximization principle that characterizes the middle eigenvalue of the matrices in parts (c-d) of Exercise 8.4.36. Exercise 8.4.36 (c) (d) 6 -4 I 1 -1 l 141 412
-
Respond to the ethical judgments required based on the following scenarios. Scenario 1. Assume you have collected a sample using MUS and that you have evaluated that sample to calculate a total...
-
Dianeackerone is a volatile natural product isolated from secretory glands of the adult African dwarf crocodile. The compound is believed to be a pheromone associated with nesting and mating....
-
Outlined here is a synthesis of glyceraldehyde (Section 5.15A). What are the intermediates A-C and what stereoisomeric form of glyceraldehyde would you expect to obtain? PCC CH3OH, HA KMnO4, HO H2o ...
-
Consider the reduction of (R)-3-phenyl-2-pentanone by sodium borohydride. After the reduction is complete, the mixture is separated by chromatography into two fractions. These fractions contain...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


