Using known reactions and mechanisms discussed in the text, complete the reactions given in Fig. P19.49. (a)
Question:
Using known reactions and mechanisms discussed in the text, complete the reactions given in Fig. P19.49.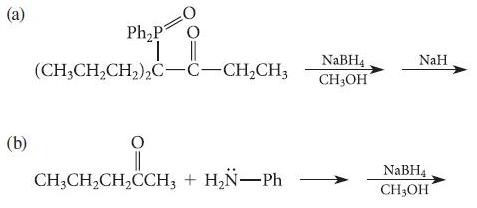
Transcribed Image Text:
(a) (b) i (CH3CH₂CH₂)₂C-C-CH₂CH3 Ph₂P i CH3CH₂CH₂CCH3 + H₂N-Ph NaBH4 CH3OH NaH NaBH4 CH3OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a In this reaction the carbonyl group is reduced to an alcohol ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using known reactions and mechanisms discussed in the text, complete the reactions given in Fig. P19.46 on p. 942. Fig. P19.46 NaBH4 CH OH
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
What are decision support systems, and what role do they play in the business environment?
-
Patel Insurance Agency reported the following items at September 30: Requirement 1. Prepare T-accounts for Patel Insurance Agency. Insert the account balances prior to closing. Post the closing...
-
The plaintiff had purchased ore from Oppenheimer. The plaintiff requested that the defendant bank negotiate documents on its behalf from Oppenheimer covering a shipment of "cobalt ore analysis not...
-
800 on the SAT. It is possible to score higher than 800 on the SAT, but scores above 800 are reported as 800. (That is, a student can get a reported score of 800 without a perfect paper.) In 2007,...
-
A jet plane at take-off can produce sound of intensity 10.0 W 1m2 at 30.0 m away. But you prefer the tranquil sound of normal conversation, which is 1.0W/m2. Assume that the plane behaves like a...
-
Instructions Valley Designs issued a 120-day, 7% note for $65,400, dated April 15 to Bork Fumiture Company on account. Required: A. Determine the due date of the note. B. Determine the matunity value...
-
(a) Complete the series of reactions in Fig. P19.50 by giving the major organic product. (b) Show how the same product could be prepared from hydroquinone monomethyl ether (p-methoxyphenol). CH0-...
-
Give the structures of the four separable isomers with the formula C 9 H 18 O 3 that are formed in the acid-catalyzed reaction of hexanal with glycerol (1,2,3-propanetriol).
-
Assume the two-dimensional structure of an ionic com-pound MxAy is What is the empirical formula of this ionic compound?
-
10.) Steam enters a well-insulated turbine at 6 MPa, 400C and expands to 200 kPa, saturated vapor at a rate of 10 kg/s. (a) Draw a schematic of the process (5 pts). (b) Determine the exergy...
-
4. [8 marks] The tides in the Bay of Fundy are some of the largest in the world. The height, h(t), of the tide in meters after t hourse can be modeled by 39 h(t) = 25 con (77) + 30 4 COS 6 (a) What...
-
Wolfe, Inc. had credit sales for the period of $144,000. The balance in Allowance for Doubtful Accounts is a debit of $653. If Wolfe estimates that 2% of credit sales will be uncollectible, what is...
-
Water at 20C is to be pumped from a reservoir (ZA = 5 m) to another reservoir at a higher elevation (ZB = 13 m) through two 36-m- long pipes connected in parallel as shown. The pipes are made of...
-
Delph Company uses a job-order costing system with a plantwide predetermined overhead rate based on machine-hours. At the beginning of the year, the company estimated that 53,000 machine-hours would...
-
Write down and solve an optimization principle characterizing the largest and smallest eigenvalue of the following positive definite matrices: 4 1 111 214 13 461 141 641 412 21
-
TRUE-FALSE QUESTIONS 1. In terms of preliminary analytical procedures, assume that the company has introduced a new product with a low price point and significant customer demand. The auditor would...
-
The structure of the sex pheromone (attractant) of the female tsetse fly has been confirmed by the following synthesis. Compound C appears to be identical to the natural pheromone in all respects...
-
Provide reagents that would accomplish each of the following syntheses. Begin by writing a retrosynthetic analysis. (a) (b) HO HO or OH
-
Write a detailed mechanism for the following reaction. OH OH H2SO4 (cat), H2O HO OH
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...
-
On an average day, a company writes checks totaling $1,500. These checks take 7 days to clear. The company receives checks totaling $1,800. These checks take 4 days to clear. The cost of debt is 9%....
-
Olds Company declares Chapter 7 bankruptcy. The following are the book values of the asset and liability accounts at that time. A bankruptcy expert estimates that administrative expense will total $...

Study smarter with the SolutionInn App


