Using known reactions and mechanisms discussed in the text, complete the reactions given in Fig. P19.46 on
Question:
Using known reactions and mechanisms discussed in the text, complete the reactions given in Fig. P19.46 on p. 942.
Fig. P19.46
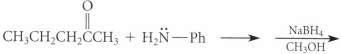
Transcribed Image Text:
NaBH4 CH OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
An initially formed imi...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Complete the reactions given in Fig. P19.45 by giving the principal organic product(s). Fig. P19.45 (a) (b) (c) p-toluenesulfonic acid (catalyst CH, t CH,OH - (solvent) ether Hio -caphenone, +...
-
Complete the reactions given in Fig. P22.81 by giving the major organic products. Explain your reasoning. NaOEt excess) EtOH H,o heat CHsI (c) CI NO, H ot, heat C CH LiAIH 2) HaO (e) CH Et CHCHCOEOH...
-
Complete the reactions given in Fig. P21.52 by giving the principal organic products. Explain how you arrived at your answers. NaOH CH O (trace) H,C CCHO CH +CH OH (solvent) Ph NH2 1 (CgH,NO3)...
-
Sedona Company set the following standard costs for one unit of its product for 2015. Direct material (20 Ibs. @ $2.50 per Ib.) . . . . . . . . . . . . . . . . . . . . . . . $ 50 Direct labor (10...
-
Cooper Grant is the president of Acme Brush of Brazil the wholly owned Brazilian subsidiary of U.S.-based Acme Brush Inc. Cooper Grants compensation package consists of a combination of salary and...
-
MNCs tend to expand more when they can more easily access funds by issuing stock. In some countries, shareholder rights are very limited, and the MNCs have limited ability to raise funds by issuing...
-
To identify the impact of e-commerce. LO.1
-
The artisans at Jewelry Junction in Phoenix are preparing to make gold jewelry during a 2-month period for the Christmas season. They can make bracelets, necklaces, and pins. Each bracelet requires...
-
The accounting equation for Golub Enterprises is as follows: Assets $720,000 = = Liabilities $360,000 + + Stockholders' Equity. $360,000 If the company now purchases office equipment on account for...
-
Fiske Corporation manufactures a popular regional brand of kitchen utensils. The design and variety have been fairly constant over the last three years. The managers at Fiske are planning for some...
-
(a) What are the two constitutionally isomeric cyclic acetals that could in principle be formed in the acid-catalyzed reaction of acetone and glycerol (1, 2, 3-propanetriol)? (b) Only one of the two...
-
A compound A, C8H8O, when treated with Zn amalgam and HCl, gives a xylene (dimethylbenzene) isomer that in turn gives only one ring monobromination product with Br2 and Fe. Propose a structure for A.
-
The SEC requires that the annual report include a. balance sheets for the two most recent yearends and income statements for each of the three most recent years. b. balance sheets for the three most...
-
9. For diatomic molecules, the correct statement(s) about the molecular orbitals formed by the overlap of two 2pz orbitals is (are) (A) orbital has a total of two nodal planes. (B) * orbital has one...
-
12. The treatment of galena with HNO3 produces a gas that is (A) paramagnetic (C) an acidic oxide (B) bent in geometry (D) colorless
-
Determine the position of the centre of gravity (c.g.) and 2nd moment of inertia with respect to c.g. of the section shown below, where A = mm and B = 5 mm. a) x B A y B Calculate the x = mm with...
-
7. Consider the design of a burglar alarm for a house. When activated an alarm and lights will be activated to encourage the unwanted guest to leave. This alarm be activated if an unauthorized...
-
A uniform flat plate of metal is situated in the reference frame shown in the figure below.
-
Agree or disagree with this statement: The variance of a portfolio is the expected value of the squared deviations of the portfolios returns from its mean return.
-
Calculate the electrical conductivity of a fiber-reinforced polyethylene part that is reinforced with 20 vol % of continuous, aligned nickel fibers.
-
Calculate the Ka for these compounds. a) HC=CH (pK = 25) b) HC N (pK = 9.31)
-
Explain which compound is the stronger acid: O a) CHCNH or CH3COH c) CHCHCHCH3 or e) CHNH or CH-NH b) CH3 SH or OH OH 6.6. & or d) CF3 CH3
-
Explain which species is the stronger base: a) :CH or :CH NO b) CH-P-CH, or CH-N-CH CH3 CH3 : : c) BICH,CO: or CICH,CO: d) CHO: or CHNH
-
How do external factors such as changing consumer preferences affect the retail industry?"
-
Production costs that are not attached to units that are sold are reported as: Cost of goods sold Selling expenses Administrative costs Inventory
-
Please show workings :) Oxford Company has limited funds available for investment and must ration the funds among four competing projects. Selected information on the four projects follows: Life of...

Study smarter with the SolutionInn App


