A compound X with the molecular formula C 5 H 10 O 2 has an IR spectrum
Question:
A compound X with the molecular formula C5H10O2 has an IR spectrum with strong absorption in the 1000–1100 cm–1 region; very strong, broad absorption in the 3000–3600 cm–1 region; and no absorption in the 1600–1700 cm–1 region. The proton NMR spectrum of X is given in Fig. P13.59. When the sample is shaken with D2O, the triplet at δ 3.5 disappears and the doublet at δ 3.7 becomes a singlet. Propose a structure for this compound, and explain your reasoning carefully.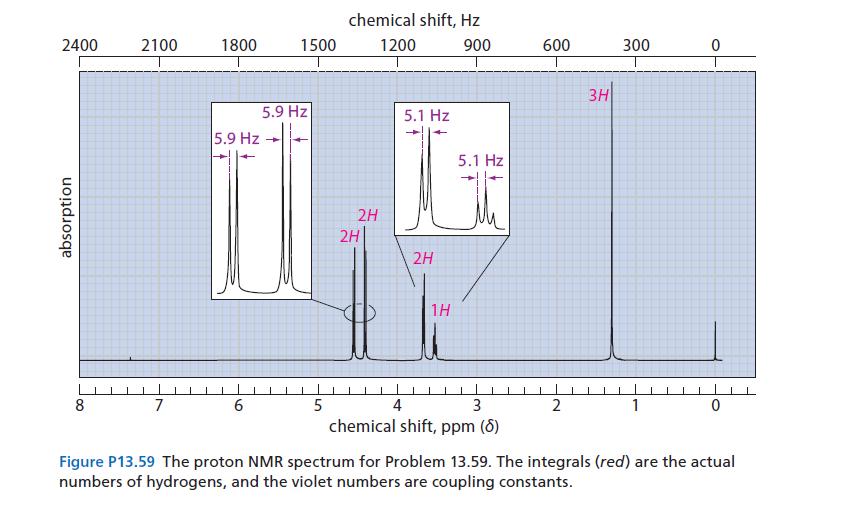
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





