(a) Draw sawhorse projections of ephedrine (Problem 6.31) about the C1C2 bond for all three staggered and...
Question:
(a) Draw sawhorse projections of ephedrine (Problem 6.31) about the C1—C2 bond for all three staggered and all three eclipsed conformations.
(b) Examine each conformation for chirality. How do the chiralities of these conformations relate to the overall chirality of ephedrine?
Problem 6.31
Ephedrine has been known in medicine since the Chinese isolated it from natural sources in about 2800 BC. Its structure has been known since 1885. Ephedrine can be used as a bronchodilator (a compound that enlarges the air passages in the lungs), but it tends to increase blood pressure because it constricts blood vessels. Pseudoephedrine has the same effects, except that it is much less active in elevating blood pressure. Ephedrine is the (1R,2S)-stereoisomer of the structure below (Ph 5 phenyl). Pseudoephedrine is the (1S,2S)-stereoisomer of the same structure.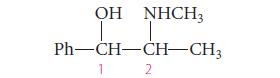
Step by Step Answer:






