Enantiomerically pure (R)-(+)-2-methyl-1,2-butanediol has a specific rotation [] 20 D + 19.3 degrees mL g 1 dm
Question:
Enantiomerically pure (R)-(+)-2-methyl-1,2-butanediol has a specific rotation [α]20D + 19.3 degrees mL g–1 dm–1 in chloroform solution.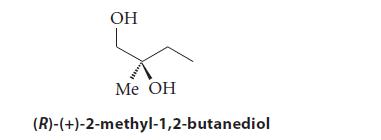
(a) What is the EE of a mixture of (+)- and (-)-2-methyl-1,2-butanediol that has an apparent specific rotation of 26.3 degrees mL g–1 dm–1 under the same conditions?
(b) What percentage of each enantiomer is present in the mixture?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





