(a) Explain why 2,4-pentanedione (Eq. 22.15) contains much less enol form in water (15%) than it does...
Question:
(a) Explain why 2,4-pentanedione (Eq. 22.15) contains much less enol form in water (15%) than it does in hexane (92%).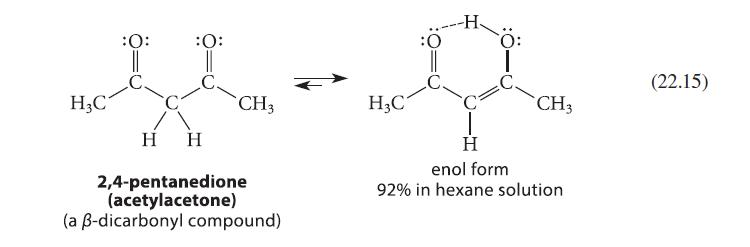
(b) Explain why the same compound has a strong UV absorption in hexane solvent (λmax = 272 nm, ϵ = 12,000), but a weaker absorption in water (λmax = 274 nm, ϵ = 2050).
Transcribed Image Text:
H₂C :O: || C. :O: H CH3 H 2,4-pentanedione (acetylacetone) (a ß-dicarbonyl compound) H3C -H Ö: CH3 H enol form 92% in hexane solution (22.15)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
a One source of stabilization of the enols of 3diketones is their intramolecular hydroge...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
For the following two graphics, provide the specified information below for each. Inverse Demand: P= 43.75 - .00625 Q; MR = 43.75 - 0.0125 Q 25 20 15 $ per unit 10 10 5 0 MC 500 1000 1500 ATC 2000 -...
-
The mass spectrum of unknown compound A shows a molecular ion at m/z 116 and prominent peaks at m/z 87 and m/z 101. Its UV spectrum shows no maximum above 200 nm. The IR and NMR spectra of A follow....
-
Julia Robertson is a senior at Tech, and she's investigating different ways to finance her final year at school. She is considering leasing a food booth outside the Tech stadium at home football...
-
The end-of-month trial balance of St. Paul Technology, Inc., at January 31, 2012, follows: Additional data at January 31, 2012: a. Supplies consumed during the month, $1,400. Half is selling expense,...
-
a. If you manage a phone assembly department, when during the month would you tend to request that your phone circuit boards be assembled by the board department (everything else being held...
-
TelComm Corporation is a manufacturer of components for the cell phone industry. TelComm founder Alex Bell heard that China has the worlds largest number of cell phone users and wants to begin...
-
The R. M. Smithers Corporation earned an operating profit margin of 10 percent based on sales of $10 million and total assets of $5 million last year. a. What was Smithers total asset turnover ratio?...
-
ow.com Mail-Syed Akhtar How To Tame AW ENO1101 Compost SCHEDULE ENGL WebWK MATI SPEECH - Google Financial statements Label and Amount Descriptions Income Statement Statement of Owner's Equity...
-
Give the products expected (if any) when each of the following compounds reacts with Br 2 in NaOH. CH3 (b) OH T PhCHCH3
-
Draw all of the enol forms of 2-butanone. Which is the least stable? Explain why?
-
Recent information for Shady Co., which makes automobile sunscreens, follows: If Shady wants to earn \($1,250\) profit next month, how many screens must it sell? a. 109. b. 136. c. 186. d. 225....
-
Write a program that solves either a) the Towers of Hanoi problem with up to 1000 disks, or, b) the Traveling Salesman problem with up to 10 cities. You may need to wait until you have read about...
-
Consider the E-R diagram in Figure 8-15b. a. What would be the identifier for the CERTIFICATE associative entity if Certificate Number were not included? b. Now assume that the same employee may take...
-
z = 1.1 for H a : < 149.6 Find the P-value that corresponds to the standard z-score, and determine whether the alternative hypothesis is supported at the 0.05 significance level.
-
An object is placed \(150 \mathrm{~mm}\) away from a converging thin lens that has a focal length of \(400 \mathrm{~mm}\). What are (a) the image distance and \((b)\) the magnification? (c) Draw a...
-
Let $M$ be the four-dimensional Minkowski space, with coordinates $x^{0}, x^{1}, x^{2}$, and $x^{3}$. Let us define a linear operator $*: \Omega^{r}(M) ightarrow$ $\Omega^{4-r}(M)$, such that...
-
Let u(t) solve Let v(r) = u(-t) be its time reversal. (a) Write down the linear system satisfied by v(t). Then classify the following statements as true or false. Explain your answers. (b) If is...
-
d. The characteristic equation of a control system is given by s+2s+8s+12s+20s+16+16=0. Determine the number of the roots of the equation which lie on the imaginary axis of s-plane
-
Give an IUPAC substitutive name for each of the following alcohols: (a) (b) (c) (d) (e) (f) OH HO HO
-
Write structural formulas for each of the following: (a) (Z)-But-2-en-1-ol (b) (R)-Butane-1,2,4-triol (c) (1R,2R)-Cyclopentane-1,2-diol (d) 1-Ethylcyclobutanol (e) 2-Chlorohex-3-yn-1-ol (f)...
-
Provide the alkene needed to synthesize each of the following by oxymercuration demercuration. (a) (b) (c) (d) OH
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...

Study smarter with the SolutionInn App


