(a) Explain why compound A has a UV spectrum with considerably greater max values and intensities...
Question:
(a) Explain why compound A has a UV spectrum with considerably greater λmax values and intensities than are observed for ethylbenzene.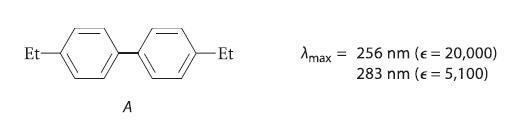
(b) In view of your answer to part (a), explain why the UV spectra of compounds B and C are virtually identical.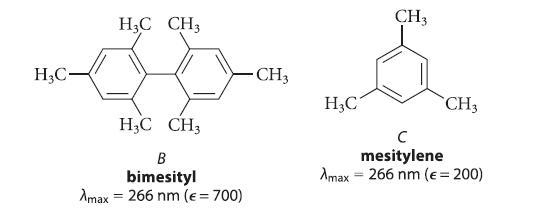
Transcribed Image Text:
Et A Et Amax 256 nm (e = 20,000) 283 nm (e 5,100) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
a The two benzene rings are conjugated in compound A because there are more bonds in conjugation the ...View the full answer

Answered By

Nimlord Kingori
2023 is my 7th year in academic writing, I have grown to be that tutor who will help raise your grade and better your GPA. At a fraction of the cost on other sites, I will work on your assignment by taking it as mine. I give it all the attention it deserves and ensures you get the grade that I promise. I am well versed in business-related subjects, information technology, Nursing, history, poetry, and statistics. Some software's that I have access to are SPSS and NVIVO. I kindly encourage you to try me; I may be all that you have been seeking, thank you.
4.90+
360+ Reviews
1070+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In a national poll of 1039 U.S. adults, conducted November 7-10, 2013, Gallup asked "Roughly how much money do you think you personally will spend on Christmas gifts this year?". The data provided on...
-
In the article Sweetening StatisticsWhat M&Ms Can Teach Us (Minitab Inc., August 2008), M. Paret and E. Martz discussed several statistical analyses that they performed on bags of M&Ms. The authors...
-
In a poll of 1009 U.S. adults of age 18 years and older, conducted December 47, 2008, Gallup asked Roughly how much money do you think you personally will spend on Christmas gifts this year?. The...
-
How are direct and indirect materials costs distinguished?
-
Relating net income to balance sheet changes Magyar T1ekom (Magyar), a Hungarian telecommunications company, reported the following balance sheet information for the years ended December 31, 2006 and...
-
Let A and B be events with P(A) = 0.5 and P(A B c ) = 0.4. For what value of P(B) will A and B be independent?
-
List the outcome(s) of the event It rains all three days.
-
In the audit of accounts receivable, auditors develop specific audit assertions related to the receivables. They then design specific substantive procedures to obtain evidence about each of these...
-
GG Products, Inc., prepares tips and stems from a joint process using asparagus. It produced 290,000 units of tips having a sales value at the split-off point of $77,000. It produced 290,000 units of...
-
A benzene derivative known to be a methyl ether with the formula C 7 H 6 OCl 2 has five lines in its proton-decoupled 13 C NMR spectrum. Propose two possible structures for this compound that fit...
-
Predict the approximate boiling point of (a) Ethylbenzene (b) Propylbenzene (c) P-xylene
-
You are a sales representative for Xerox Canada, which has just designed a new, less expensive, and better quality copying machine. From the material in this chapter, identify the sources you would...
-
The ratio of CEO pay to that of an average employee increased over a period of 50 years from 24:1 to 275:1. Is this increasing gap ethically sound, in your opinion? Should CEO pay be limited in any...
-
Suppose you are considering buying a machine that costs $7,000. It will generate revenues of $1,500 for the next 3 years, and then $1,000 for the following 5 years. What is the payback period of this...
-
National Bakery Limited is the main supplier of a variety of baked products to customers in Kingston. The company currently makes 25,000,000 a variety of baked products annually which uses baking...
-
Q1. Discuss the financial goal of a business. Ensure to provide an example of the inherent ethical challenges associated with the financial goal and or the financial management process. Using the...
-
Q1. How can companies use social media to do sentiment analysis? Describe the process. Give an example of a company that uses sentiment analysis to enhance relationships with customers. Q2. Describe...
-
Miller Mining acquired rights to a tract of land with the intent of extracting from the land a valuable mineral. The cost of the rights was $2,500,000 and an estimated 10,000 tons of the mineral are...
-
What kind of financial pressures can an LBO cause?
-
Rank the following compounds in order of increasing reactivity (least reactive first) in an SN1 solvolysis reaction in aqueous acetone. Explain your answers. (The structure of tert-cumyl chloride is...
-
Explain why compound A reacts faster than compound B when they undergo solvolysis in aqueous acetone. CH C-Cl CH3 CH CH
-
A hydrocarbon A, C9H12, is treated with A-bromosuccinimide in CCl4 in the presence of peroxides to give a compound B, C9H11Br. Compound B undergoes rapid solvolysis in aqueous acetone to give an...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...
-
Pedro lives in Puerto Rico and had a net taxable income of $35,000 for the year 20X1. Your gross income totals $60,000. What is Pedro's regular income tax for 20X1? a.$4,620 b.$4,900 c.$2,318 d.$2,520
-
The change in cash is equal to the change in liabilities less the change in equity plus the change in noncash assets. O True False

Study smarter with the SolutionInn App


