(a) How might the structure of the resin in Eq. 27.9 be altered to make the resin...
Question:
(a) How might the structure of the resin in Eq. 27.9 be altered to make the resin an anion exchanger (that is, an anion-binding resin)?
(b) Predict the order of elution of the following peptides from an anion-exchange resin at pH 6: A-V-G, D-E-E-G, D-N-N-G.
Explain your reasoning.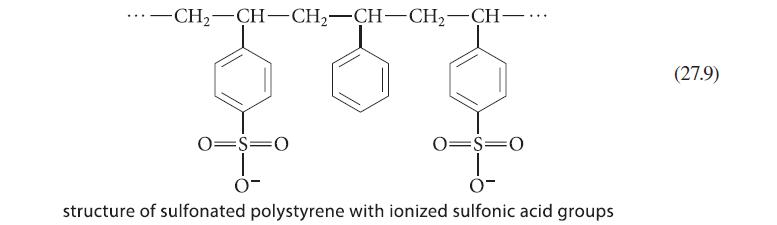
Transcribed Image Text:
-CH2-CH-CHz-CH-CH2-CH一… 0-S-0 O- 08-0 structure of sulfonated polystyrene with ionized sulfonic acid groups (27.9)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a A resin containing a cationic group will serve as ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How might the structure of the environment; for example, the distributions of different soil types and soil moisture, affect the patterns of distribution in plant populations? How should interactions...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The doctrine of intention to create legal relations has now become a challenge towards the doctrine of consideration, which has been riddled with so many criticisms and as such the former doctrine...
-
The comparative financial statements prepared at December 31, 2012, for Prince Company showed the following summarized data: *One-third was credit sales. During 2012, cash dividends amounting to...
-
You make $17,500/year. You pay $100 in gasoline tax, which is the same amount your parents pay. a. What percentage of your income goes towards this gasoline tax? b. Is this a regressive or...
-
What is the difference between a marketing dashboard and a marketing metric? LO.1
-
Review each of the following independent sets of conditions. For each condition, calculate the (1) Sample rate of deviation, and use AICPA sample evaluation tables to identify the (2) Upper limit...
-
The value of a put option decreases as the stock price increases, all other things equal. True False
-
(a) Estimate the isoelectric point of each of the following peptides. A-K-V-I-M G-D-G-L-F (b) Draw the structures of these peptides, indicating the predominant ionization state of each at its...
-
(a) What is the -carbon configuration of L -cysteine in the R,S system? (b) Explain why L -cysteine and L -serine have different configurations in the R,S system.
-
What ethical considerations are important in development of technology in general, as well as AI (artificial intelligence)?
-
Pop Company holds 70% of Son Company stock. Pop has sold inventory to Son Company as follows: Percent of Sold Sales Inventory Cost to Price to Held at Year Pop Son Year end 2018 $203,000 $355,000 30%...
-
A B C D E F G H J K L 1 Cost Mortgage Payments 2 Cost Description The upscale hotel's building was acquired for $10 million, leading to monthly mortgage payments of $60,000. Behavior Dollar Amount...
-
What celebrity attributes make for effective celebrity product endorsements? Celebrity testimonials are advertising messages delivered by famous people who say or imply that they use the...
-
Initial investment of $100,000 in a new medical equipment. Interest Rate 10% (Borrowed money from a bank). Item Year 0 Year 1 Year 2 Year 3 Year 4 Year 5 Investment 100,000 Expected Cash Flow 20,000...
-
How do I draw a top view of this sketch and I was also wondering how to draw an oblique cabinet projection. + + 1 1 ' '
-
Raneri Trophies Inc. uses a job order cost system for determining the cost to manufacture award products (plaques and trophies). Among the company's products is an engraved plaque that is awarded to...
-
l ask this second time correnct answer is 38,01 can we look pls Consider a non-conducting rod of length 8.8 m having a uniform charge density 4.5 nC/m. Find the electric potential at P, a...
-
A widely used undergraduate experiment is the recrystallization of acetanilide from water. Acetanilide (see following structure) is moderately soluble in hot water, but much less soluble in cold...
-
Give the structure of each of the following compounds. (a) Chlorocyclopropane (b) Methylene iodide
-
Acetone (Table 8.2) has a significant dipole moment (2.7 D). Using structures, show the stabilizing interactions to be expected between acetone solvent molecules and (a) A dissolved potassium ion;...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


