A student has run the reactions shown in Fig. P11.51 and is disappointed to find that each
Question:
A student has run the reactions shown in Fig. P11.51 and is disappointed to find that each has given none of the desired product. Explain why each reaction failed.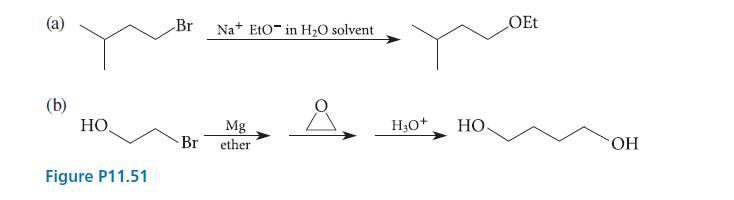
Transcribed Image Text:
(b) HO, Figure P11.51 Br Na EtO in H₂O solvent Mg Br ether H3O+ HO OEt OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a b Sodium ethoxide reacts with water to give ethanol and sodium hydroxide Although the pK ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A student who has created a linear model is disappointed to find that her R2 value is a very low 13%. a) Does this mean that a linear model is not appropriate? Explain. b) Does this model allow the...
-
Leadership in Organizations Module Three Assignment Essay #1: Case Study: Echo Electronics Paul Sanchez is the production manager for Echo Electronics, a small company that makes and distributes...
-
An intern who has created a linear model is disappointed to find that her R2 value is a very low 13%. a) Does this mean that a linear model is not appropriate? Explain. b) Does this model allow the...
-
The preparation of an organization's budget: a. forces management to look ahead and try to see the future of the organization. b. requires that the entire management team work together to make and...
-
Suggest reasons why the total assets and total liabilities of a defined benefit pension plan do not appear, but their net amount does appear, on the employers balance sheet.
-
During December, the company completed the following summary transactions. Dec. 6 Paid $1,600 for salaries due employees, of which $600 is for December and $1,000 is for November salaries payable....
-
What specific factors contribute to holding period premiums?
-
The Caribbean nations do not participate in NAFTA and CAFTA-DR. Many people in southern U.S. states complain that NAFTA and CAFTA-DR are unfair to their extended families living on the Caribbean...
-
The Sellers Affidavit of Nonforeign Status (AS) form, by which the seller provides a signed, sworn oath that he or she is not a nonresident alien is required to avoid withholding requirements of...
-
When HCl is formed as a by-product in reactions, it is usually removed from reaction mixtures by neutralization with aqueous base. At times, however, the use of base is not compatible with the...
-
Give the major organic product of each of the following reactions. Include stereochemistry where relevant. (a) Dibutyl sulfide with 1 equivalent of H 2 O 2 at 25C (b) Dibutyl sulfide with 2 or more...
-
Laminar flow in a triangular duct (Figure 3B.2) 2 one type of compact heat exchanger is shown in Figure 3B.2 (a). In order to analyze the performance of such an apparatus, it is necessary to...
-
A production Edgeworth Box, with origins indicated for the inputs of capital, K , and labor, L , into production of goods X and Y .Eight isoquants are shown, reflecting standard...
-
For 2014, Nichols, Inc., had sales of 150,000 units and production of 200,000 units. Other information for the year included: Direct manufacturing labor 187,500 Variable manufacturing overhead...
-
reading the following statement and decide whether you agree or disagree with the statement: "The free market system is the best economic system since it is the most efficient and solves basic...
-
find the net presbf value of the project ? present value index? Net present value A project has estimated annual net cash flows of $11,250 for 10 years and is estimated to cost $42,500. Assume a...
-
Calculate the ICER for the new treatment, without adjusting for the health utility index. Assuming the $50K benchmark*, as a clinical decision maker or health policy advisor, would you recommend...
-
Evaluate without using a calculator. (a) Tan /6 (b) Sec (c) Sec 3/4 (d) Csc /2 (e) Cot /4 (f) Tan (-/4)
-
Complete problem P10-21 using ASPE. Data from P10-21 Original cost ................................................................. $7,000,000 Accumulated depreciation...
-
Which amide bonds in the following polypeptide are cleaved by trypsin by chymotrypsin? Phe-Leu-Met-Lys-Tyr-Asp-Gly-Gly-Arg-Val-IIe- Pro-Tyr
-
What kinds of reactions do the following classes of enzymes catalyze? (a) Hydrolases (b) Lyases (c) Transferases
-
Which of the following amino acids are more likely to be found on the outside of a globular protein, and which on the inside? Explain. (a) Valine (b) Aspartic acid (c) Phenylalanine (d) Lysine
-
Ted and his partners have contracted to purchase the franchise nights worth 561 000 to open and operate a specialty pizza restaurant called Popper with a renewable agrement, the partners have agreed...
-
Your answer is partially correct. Martin Company's chief financial officer feels that it is important to have data for the entire quarter especially since their financial forecasts indicate some...
-
Kellog Corporation is considering a capital budgeting project that would have a useful life of 4 years and would love testing 5156.000 in equipment that would have zeto salvage value at the end of...

Study smarter with the SolutionInn App


