(a) The relatively stable carbocation crystal violet has a deep blue-violet color in aqueous solution. When NaOH...
Question:
(a) The relatively stable carbocation crystal violet has a deep blue-violet color in aqueous solution. When NaOH is added to the solution, the blue color fades because the carbocation reacts with sodium hydroxide in about 1–2 min to give a colorless product.
Show the reaction of crystal violet with NaOH and explain why the color changes.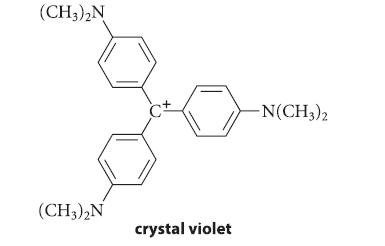
(b) When the detergent sodium dodecyl sulfate (SDS) is present in solution above its critical micelle concentration, the bleaching of crystal violet with NaOH takes several days. Account for the effect of SDS on the rate of this bleaching reaction.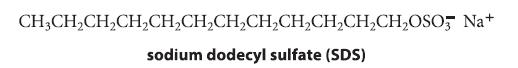
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





