(a) What value is expected for the dipole moment of the anti conformation of 1, 2-dibromoethane, BrCH...
Question:
(a) What value is expected for the dipole moment of the anti conformation of 1, 2-dibromoethane, Br—CH2—CH2—Br? Explain.
(b) The dipole moment μ of any compound that undergoes internal rotation can be expressed as a weighted average of the dipole moments of each of its conformations by the following equation: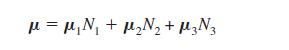
in which μi is the dipole moment of conformation i, and Ni is the mole fraction of conformation i. (The mole fraction of any conformation i is the number of moles of i divided by the total moles of all conformations.)
There are about 82 mole percent of anti conformation and about 9 mole percent of each gauche conformation present at equilibrium in 1,2-dibromoethane, and the observed dipole moment μ of 1,2 dibromoethane is 1.0 D. Using the preceding equation and the answer to part (a), calculate the dipole moment of a gauche conformation of 1,2-dibromoethane.
Step by Step Answer:






