From what you learned in Sec. 1.3B about the relative lengths of CC and CO bonds, predict
Question:
From what you learned in Sec. 1.3B about the relative lengths of C¬C and C¬O bonds, predict which of the following compounds should have the larger energy difference between gauche and anti conformations about the indicated bond. Explain.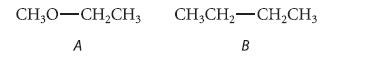
Transcribed Image Text:
CH3O-CH₂CH3 CHỊCH,—CH,CH3 A B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The CO bond is somewhat shorter than the CC bond text p 15 ...View the full answer

Answered By

Elias Gichuru
am devoted to my work and dedicated in helping my clients accomplish their goals and objectives,providing the best for all tasks assigned to me as a freelancer,providing high quality work that yields high scores.promise to serve them earnestly and help them achieve their goals.i have the needed expertise,knowledge and experience to handle their tasks.
4.80+
325+ Reviews
859+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Do HF/6-31G* partial geometry optimizations of n-butane conformations with CCCC dihedral angles fixed at several values. Plot the energy versus dihedral angle. From the plot, estimate the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Which one of the following compounds should have a * UV absorption at the greater max when the compound is dissolved in NaOH solution? Explain.
-
The task in this design project is to design an actuation system to power the ram's reciprocating motion in a small-size shaper. The power source of the actuation system is an AC motor with 0.75 hp...
-
How can a judge decide whether there was sexual harassment without holding a trial? We dont know whether Clinton did these things or not.
-
Acetonitrile (CH3CN) is a polar organic solvent that dissolves a wide range of solutes, including many salts. The density of a 1.80 M LiBr solution in acetonitrile is 0.826 g/cm3. Calculate the...
-
5. Refer to Exhibit 15.6, Part B from Consumer Products Cos annual report. Here you will find financials by segment. What are the operating margins by segment? Based on operating margin by segment,...
-
The Excel file golf scores. xlsx contains a random sample of golf scores from two highly competitive sons of a certain statistics author. Each son claims he is the better golfer. Assume the...
-
Required: 1. Determine the par values of the corporation's preferred stock and its common stock
-
(a) What value is expected for the dipole moment of the anti conformation of 1, 2-dibromoethane, BrCH 2 CH 2 Br? Explain. (b) The dipole moment of any compound that undergoes internal rotation can...
-
(a) Draw Newman projections of the most stable conformations about each of the carboncarbon bonds in the principal chain of 2,2-dimethylpentane. Use models! (b) Combine these to predict the most...
-
On March 31, 2014, Taggert Company had a cash balance per books of $5,174.20. The statement from Western Bank on that date showed a balance of $6,041.40. A comparison of the bank statement with the...
-
What are major initiatives would you expect to see in a strategic plan focusing on a public health organization?
-
The purchase of \(\$ 500\) of supplies on account will: a. Increase both assets and stockholders' equity by \(\$ 500\) b. Increase assets and decrease liabilities by \(\$ 500\) c. Increase assets and...
-
Venus Company owned a service truck that was purchased at the beginning of 2011 for \(\$ 20,000\). It had an estimated life of three years and an estimated salvage value of \(\$ 2,000\). Venus uses...
-
You are observing the sales department staff using exponential smoothing to fore- cast monthly sales. Their forecast for January's sales was 12,000 units. January's actual sales figure became...
-
Use the ID3 algorithm to build the full decision tree for the data set given in Section 10.9.2. 10.9.2 Example We will start with the training data given below: Film Country of origin Big star Genre...
-
List the major organs that compose each organ system and identify their functions.
-
Why is it important to understand the macro-environment when making decisions about an international retail venture?
-
Propose structures for molecules that meet the following descriptions. Assume that the kinds of carbons (1, 2, 3, or 4) have been assigned by DEPT-NMR. (a) C 6 H 12 O; IR: 1715 cm 1 ; 13 C NMR: 8.0 ...
-
Compound A, C 8 H 10 O 2 , has an intense JR absorption at 1750 cm ?1 and gives the 13 C NMR spectrum shown. Propose a structure for A. 219 8 TMS 200 180 160 140 120 40 O ppm 80 60 20 100 Chemical...
-
Propose structures for ketones or aldehydes that have the following 1 H NMR spectra: (a) C 4 H 7 C1O ??IR: 1715 cm ?1 ? (b) C 7 H 14 O ? ? ?IR: 1710 cm ?1 ? (c) C 9 H 10 O 2 ? ? ?IR: 1695 cm ?1 ?...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


