(a) Which of the compounds shown in Fig. P6.40 can in principle be resolved into enantiomers? Explain...
Question:
(a) Which of the compounds shown in Fig. P6.40 can in principle be resolved into enantiomers? Explain why or why not.
(b) Omeprazole can be separated into two enantiomers that do not interconvert at room temperature.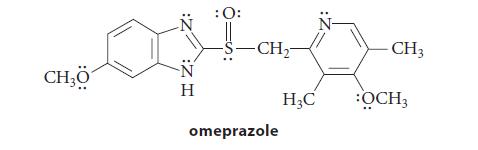
(c) Esomeprazole, the S enantiomer of omeprazole, is a drug used to control acid reflux. Redraw the structure of omeprazole in part (b) to show it as the S enantiomer.
Transcribed Image Text:
CH₂0 :Z H Η : 0: -S-CH₂- omeprazole H₂C - CH3 :OCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a Any compound that is chiral can in principle be resolved into enantiomers Compound A possesses a p...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds could in principle be resolved into enantiomers at very low temperatures? Explain. (a) Propane (b) 2,2,3,3, -tetramethylbutane
-
Which of the following compounds could be resolved into enantiomers at room temperature? Explain. (a) (b) CH2CH CH3)2 CH CH CH3 CH3
-
Glorious Electrical Appliances (GEP) Co. is a company that sells electrical tools. GEP uses perpetual inventory system in recording its inventory. The financial position of GEP as at 31 December 2016...
-
The Ramirez Company current dividend was $1.90. Its dividend will grow by 6%, 7%, 8% and 9% for the first 4 years. And then, dividends to grow at a rate of 5% forever. It's required return is 15%. 1-...
-
Make an argument that what McElveen and friends did indicates nothing immoral about them but rather something wrong with society.
-
Assume that the deflected shape of a beam AB with immovable pinned supports (see figure) is given by the equation v = δ sin Ï x/L, where δ is the deflection at the...
-
A company working toward JIT will have smaller lot sizes when compared to using traditional methods. Discuss how this will affect the costs associated with inventory. What are the controllable and...
-
Sean McNamee was the owner of an accounting firm, W. F. McNamee & Co., LLC (WFM), which he founded and formed in Connecticut as a limited liability company (LLC). For federal tax purposes, an LLC can...
-
X Company, a merchandiser, had the following transactions in August: -Borrowed $30,000 from a bank. -Bought equipment costing $10,000, paying the manufacturer $6,000 in cash and promising to pay the...
-
The specific rotation of the R enantiomer of the following alkene is [a] 25 D 5 179 degrees mL g 1 dm 1 , and its molecular mass is 146.2. (a) What is the observed rotation of a 0.5 M solution of...
-
Construct sawhorse and Newman projections (Sec. 2.3A) of the three staggered conformations of 2-methylbutane (isopentane) that result from rotation about the C2C3 bond. (a) Identify the conformations...
-
Describe the role that accrual accounting information and cash flow information play in your own models of company valuation.
-
Jason is a sole trader in the architecture industry. For the year ending 30 June 2019, Jason hired a 3D model designer, Sarah, to help him with the growing business. At the end of the year he has the...
-
Read Case 14-1 Trojan Technologies (15th ed., p. 426 OR 16th ed., p. 431) Guiding Questions and additional information: In preparing your case study, ensure that you answer the following questions:...
-
Jorge Rimert works for Road to Success Collection Agency. He oversees mailing out collection notices to patients. Upon review of the patients who have not paid from Hideaway Hospital, Jorge notices...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 99% confident that the estimated percentage is in error...
-
A 100 acre plot of land has a concentration time of 80 minutes. The area is residential single family homes with a C-0.40. What is the percent Increase in stormwater runoff from a 50 year recurrence...
-
Explain the function of a synovial membrane.
-
The May 2014 revenue and cost information for Houston Outfitters, Inc. follow: Sales Revenue (at standard).............. $ 540,000 Cost of Goods Sold (at standard) ..........341,000 Direct Materials...
-
Predict the major product(s) of nitration of the following substances. Which react faster than benzene and which slower? (a) Bromobenzene (b) Benzonitrile (c) Benzoic acid (d) Nitrobenzene (e)...
-
Rank the compounds in each group according to their reactivity toward electrophilic substitution. (a) Chlorobenzene, o-dichlorobenzene, benzene (b) p-Bromonitrobenzene, nitrobenzene, phenol (c)...
-
A Predict the major mono-alkylation products you would expect to obtain from reaction of the following substances with chloromethane arid AlCl3: (a) Bromobenzene (b) m-Bromo-phenol (c)...
-
You are evaluating a new project for the firm you work for, a publicly listed firm. The firm typically finances new projects using the same mix of financing as in its capital structure, but this...
-
state, "The subscription price during a rights offering is normally r; lower ; lower r; higher er; higher than the rights-on price and
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...

Study smarter with the SolutionInn App


